Anatomy is a subdiscipline of morphology concerned with naming and describing the structure of organisms based on gross observation, dissection, and microscopical examination. Morphology and anatomy are not synonyms. Morphology is concerned with the form and function of anatomical structure; because anatomy is an expression of organic evolution, morphology seeks to investigate possible explanations for organic diversification observed in nature. Before 1940, insect morphology focused on naming and describing anatomical structure. The need for this activity has not diminished, as there is much about insect anatomy remains to be revealed, described, and understood. This article focuses on the anatomical structures of the three major tagmata of the insect body: head, thorax, and abdomen, and on the external genitalia. A hypothetical ground plan for major structures is given, followed by themes in anatomical variation based on adaptation observed in the Insecta.
CONTEXT OF ANATOMICAL STUDY
Terms of Orientation and Conventions
Terms to describe orientation are not intuitive for insects. Most orientation terms are derived from the study of the human body—a body that stands upright—and their application to insects causes confusion. Some standard terms used with insects include anterior (in front), posterior (behind), dorsal (above), ventral (below), medial (middle), and lateral (side). Anatomical description usually follows in the same order; hence, we begin our discussion with the head, move on to the thorax and then the abdomen, and finish with the genitalia. Description of the relative placement of anatomical features can be cumbersome, but they are critical elements in the study of anatomical structure because relative position is one of the three basic tenets of homology, including size, shape, and embryology.
Measures of Success
The design of the insect body can be described as successful for many reasons: there are millions of species, they range in size over four orders of magnitude, their extensiveness of terrestrial and aquatic habitat exploitation (the diversity of resources), and once a successful form has been developed, there appears to be relatively little change over evolutionary time (Fig. 1). The basic insect design allows for adaptation to a variety of environmental requirements. The success of the design is rooted in the nature of the main material used for its construction.
The Building Material
When we look at an insect, it is the integument that we see. Structurally, the integument is a multiple-layered, composite organ

FIGURE 1 Fossil insects are easily recognizable today, indicating an early establishment of a successful design. Left to right: Heplagenes (Late Jurassic 150 mya, Liaoning, China); cricket (Eocene, 50 mya, Green River formation, Utah); fulgorid (Eocene, 50 mya, Green River formation, Utah).
that defines body shape, size, and color. The ultrastructure of the integument is composed of living cells and the secretory products of those cells. Each layer is of a different thickness and chemical composition, and each displays physical properties different from those of the surrounding layers. Perhaps more importantly, the integument is also the organ with the greatest diversity of structure and function.
There are two common misconceptions about the integument. First, some believe chitin is responsible for integument in the soft and flexible membranous parts of the integument than in the hard, sclerotized plates. Integument hardness is attributed to an increased number of cross-linkages between protein chains contained in the integument layers. Second, some believe that the integument is rigid and that growth is incremental and limited to expansion during molting; yet, some endopterygote insects are able to grow continuously between molts.
The integument determines the shape of the insect body and its appendages. One of the most captivating features of insects is their seemingly infinite variation in body shapes—everything from a simple bag (Hymenoptera grub) to a mimic of orchid flowers (Mantidae). Similarly, appendage shape is exceedingly plastic. Terms such as “pectinate,” “flabbate,” and “filiform” are among more than 30 terms taxonomists have proposed to describe antennal shapes. Leg shapes are similarly highly variable and express functional modifications. Among these shapes are “cursorial,” “gressorial,” “raptorial,” “fossorial,” and “scansorial.” Again, these modifications of shape reflect the function of structure. Finally, wing shapes are highly variable among insects and are determined by body size and shape as well as by aerodynamic considerations.
Tagmata
Most people recognize the three tagmata—head, thorax, and abdomen—as characteristic of insects. The way they appear is rooted in a division of responsibilities. The head is for orientation, ingestion, and cognitive process; the thorax for locomotion; and the abdomen for digestion and reproduction. But even casual observations reveal further divisions of these body regions.
Segmentation of the Tagmata
Two types of segmentation are evident among arthropods, primary and secondary. Primary segmentation is characteristic of soft-bodied organisms such as larval holometabolans. The body wall in these organisms is punctuated by grooves or rings that surround the anterior and posterior margins of each somite. These rings represent intersegmental lines of the body wall and define the limits of each somite. Internally, the grooves coincide with the lines of attachment of the primary longitudinal muscles. From a functional standpoint, this intrasegmental, longitudinal musculature permits flexibility and enables the body to move from side to side.
More complex plans of body organization exhibit structural modifications. Secondary segmentation is characteristic of hard-bodied arthropods, including adult and nymphal insects. Secondary body segmentation is an evolutionarily derived anatomical feature. The musculature we see in secondary segmentation is intersegmental, or between segments (Fig. 2) . The acquisition of secondary segmentation represents a major evolutionary step in the development of the Arthropoda. The soft-bodied arthropod has primary segmentation and muscles that are i ntrasegmental, or within each segment. Movement of the body and its parts is relatively simple because the body wall is flexible. However, when the body wall becomes hardened, flexibility is restricted to the articulation between hardened parts or the extension provided by intersegmental membranes. The arthropod is, in a metaphorical sense, clad in a suit of armor; most movement is possible only if soft and flexible membranes are positioned between inflexible (hardened) body parts. Exceptions may be seen in the indirect flight mechanism of pterygote insects.

FIGURE 2 Secondary segmentation. Diagram of sagittal section of dorsal sclerites of thorax.
In all probability secondary segmentation evolved many times, and it probably continues to evolve in response to specific problems confronting insects today. Secondary segmentation is most evident and most readily appreciated in the insect abdomen. It is less apparent in the thorax and almost totally obscured in the head.
Sclerites
The hardening of the body wall contributes significantly to the external features observed in insects. Sclerites are hardened areas of the insect body wall that are consequences of the process of sclero-tization. Sclerites, also called plates, are variable in size and shape. Sclerites do not define anatomical areas and do not reflect a common plan of segmentation. Sclerites develop as de novo hardening of membranous areas of the body wall, as de novo separations from larger sclerotized areas of the body, and in other ways.
The hardened insect body displays many superficial and internal features that are a consequence of hardening. Understanding the distinction between these conditions and the terms applied to them is critical in understanding insect anatomy and its application in taxonomic identification. These features are of three types. First, sutures (Latin, sutura = seam), in the traditional sense of vertebrate anatomists, provide seams that are produced by the union of adjacent sclerotized parts of the body wall. On the insect body, sutures appear as etchings on the surface of the body and form lines of contact between sclerites. Second, sulci (Latin, sulcus = furrow) represent any externally visible line formed by the inflection of cuticle. Biomechanically, a sulcus forms a strengthening ridge. In contrast, lines of weakness are cuticular features that are used at molting. Lines of weakness are frequently named as if they were sutures, but they should not be viewed as such. For instance, the ecdysial cleavage line is a line of weakness that is sometimes considered to be synonymous with the epicranial suture. The two features are similar in position and appearance, but structurally they may have been derived from different conditions. Finally, apodemes (Greek, apo = away; demas = body) are hardened cuticular inflections of the body wall that are usually marked externally by a groove or pit. Structures called apophyses (Greek, apo = away; phyein = to bring forth) are armlike apodemes. Apodemes have been defined as a hollow invagi-nation or inflection of the cuticle and an apophysis as a solid invagi-nation. Functionally, apodemes strengthen the body wall and serve as a surface for muscle attachment.
Sclerites receive different names depending upon the region of the body they are located. Tergites (Latin, tergum = back) are scle-rites that form a subdivision of the dorsal part of the body wall (ter-gum). Latrotergites are sclerites that form as a subdivision of the lateral portion of the tergum. Sternites (Latin, sternum = breast bone) are sclerites that form as a subdivision of the ventral part of the body wall (sternum), or any of the sclerotic components of the definitive sternum. Pleurites (Greek, pleura = side) are sclerites in the pleural region of the body wall that are derived from limb bases.
HEAD
The head is a controversial area for anatomical nomenclature, but it provides some of the best examples of evolutionary trends in anatomy. Most insect morphologists believe that the head of modern insects represents the fusion of several segments that were present in an ancestral condition. However, the number of segments included in the ground plan of the insect head has been a contentious issue among morphologists for more than a century. Any argument that attempts to explain head segmentation must take into account comparative anatomical, embryological, and paleontological evidence, and must examine modern forms of ancestral insects.
Ground Plan of the Pterygote Head
Given the difficulty in homologizing anatomical features of the head, we describe regions associated with landmarks of a ground plan or an idealized hypognathous insect head. In terms of modern insects, the Orthoptera probably come closest to displaying all the important landmark sutures and sclerites that form the head (Fig. 3A ).
The vertex (Latin, vertex = top; pl., vertices) is the apex or dorsal region of the head between the compound eyes for insects with a hypognathous or opisthognathous head. This definition does not apply to prognathous heads because the primary axis of the head has rotated 90° to become parallel to the primary axis of the body. The vertex is the area in which ocelli are usually located. In some insects this region has become modified or assumes different names.
The ecdysial suture (coronal suture + frontal suture, epicranial suture, ecdysial line, cleavage line) is variably developed among insects. The suture is longitudinal on the vertex and separates epicranial halves of the head (Fig. 3B). Depending on the insect, the ecdysial suture may be shaped like a Y, a U, or a V. The arms of the ecdysial suture that diverge anteroventrally, called the frontal sutures (frontogenal sutures), are not present in all insects (Fig. 3B). Some of these complexes of sutures are used by insects to emerge from the old integument during molting.
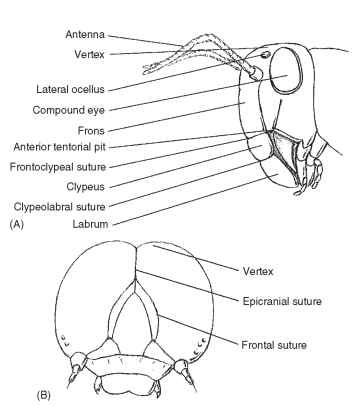
FIGURE 3 (A) Anterior view of the head of a grasshopper (Orthoptera: Acrididae). (B) Larval pterygote head showing epicra-nial and frontal sutures (Lepidoptera: Noctuidae).
The frons is that part of the head immediately ventrad of the vertex (Fig. 3A). The frons varies in size, and its borders are sometimes difficult to establish. In most insects the frons is limited ventrally by the frontoclypeal suture (epistomal suture), a transverse suture located below the antennal sockets. As its name implies, the suture separates the dorsal frons from the ventral clypeus (Fig. 3A).
The face is a generalized term used to describe the anteromedial portion of the head bounded dorsally by the insertion of the antennae, laterally by the medial margins of the compound eyes, and ven-trally by the frontoclypeal suture. In some insects the area termed the face is coincident with some, most, or all of the frons.
The clypeus (Latin, shield) is a sclerite between the face and labrum (Fig. 3A). Dorsally, the clypeus is separated from the face or frons by the frontoclypeal suture in primitive insects. Laterally, the clypeogenal suture demarcates the clypeus. Ventrally, the clypeus is separated from the labrum by the clypeolabral suture (Fig. 3A). The clypeus is highly variable in size and shape. Among insects with sucking mouthparts, the clypeus is large.
The gena (Latin, cheek; pl., genae) forms the cheek or sclero-tized area on each side of the head below the compound eye and extending to the gular suture (Fig. 4). The size of the gena varies considerably, and its boundaries also often are difficult to establish. In Odonata the gena is the area between compound eye, clypeus, and mouthparts. The postgena (Latin, post = after; gena = cheek; pl., postgenae) is the portion of the head immediately posteriad of the gena of pterygote insects and forms the lateral and ventral parts of the occipital arch (sensu Snodgrass) (Fig. 4). The subgenual area is usually narrow, located above the gnathal appendages (mandible and maxillae), and includes the hypostoma (Figs. 3 and 4 ) and the pleurostoma. The pleurostoma is the sclerotized area between the anterior attachment of the mandible and the ventral portion of the compound eye. The hypostoma is posteriad of the pleurostoma between the posterior attachment of the mandible and the occipital foramen. The subgenal suture forms a lateral, submarginal groove or sulcus on the head, just above the bases of the gnathal appendages (Fig. 4). The subgenal suture is continuous anteriorly with the frontoclypeal suture in the generalized pterygote head. Internally, the subgenal suture forms a subgenal ridge that presumably provides structural support for the head above the mandible and maxillae. In some instances, the subgenal suture is descriptively divided into two. The part of the suture that borders the proximal attachment of the mandible to the head (Fig. 4) is called the pleurostomal suture (the ventral border of the pleurostoma). The posterior part of the subge-nal suture from the mandible to the occipital foramen is called the hypostomal suture (the ventral border of the hypostoma).
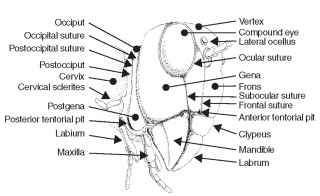
FIGURE 4 Generalized view of an insect head.
Head Size and Shape
The size and shape of the head and its appendages reflect functional adaptations that can be used to explain biological details of the insect—the realm of morphology as opposed to anatomy.
SIZE Upon casual observation, the size of any given insect’s head appears to be in proportion to the size of its body. A head that is disproportionately small or large relative to body size suggests that some adaptation has taken place that serves a functional need. Proportional head size varies considerably in the Insecta. Some fly families have very tiny heads in relation to their body size (e.g., Diptera: Acroceridae). Among Orthoptera, grass-feeding species typically have larger heads than herbaceous-feeding species. The large head is filled with powerful adductor muscles because grasses (monocots) are more difficult to chew than dicotyledonous plants. Furthermore, the postseedling stages of grasses are nutrient poor, meaning that more grass must be bitten, chopped, or ground to provide adequate nutrition.
SHAPE Head shape varies considerably among insects. Many unusual shapes seem to be influenced by behavior and may be used to illustrate examples of structural form and function. The functional importance of head shape may be difficult to determine in preserved specimens. A few hours of observation with live insects can provide considerable insight into the importance of shape. Globular heads are seen in some insects, including the burrowing crickets (e.g., stenopelmatines and gryllids). This form of head is adapted for pushing soil. Hypercephalic heads are seen in the males of some Diptera (Sepsidae, Diopsidae, Drosophilidae, and Tephritoidea) and
Hymenoptera (Pteromalidae and Eurytomidae); the broad heads of the males are featured in various aspects of courtship behaviors.
Topographical Features of the Head
Morphologists experience considerable difficulty in defining regions and determining homologies of structure on the insect head. We cannot unambiguously characterize topographical features of the insect head because more than a million species are involved in the definition, and they show incredible diversity in head anatomy. Shape alone is not adequate or suitable because there are many head shapes, and often a head shape can be derived independently in several unrelated lineages. Some head shapes are influenced by behavior.
AXIAL POSITION The posture or orientation of the head in its resting position relative to the long axis of the body can be important in providing definitions of the anatomical features of the head. Axial position in insects typically falls into three basic categories: hypognathous, prognathous, and opisthognathous.
In general zoological usage, the word “hypognathous” (Greek, hypo = under; gnathos = jaw) serves to designate animals whose lower jaw is slightly longer than the upper jaw. In entomological usage, “hypognathous” refers to insects with the head vertically oriented and the mouth-directed ventrad. Most insects with a hypogna-thous condition display an occipital foramen near the center of the posterior surface of the head. The hypognathous condition is considered by most insect morphologists to represent the primitive or generalized condition. The hypognathous position is evident in most major groups of insects and can be seen in the grasshopper, house fly, and honey bee. Other conditions are probably derived from ancestors with a hypognathous head.
In general zoological usage “prognathous” (Greek, pro = forward; gnathos = jaw) refers to animals with prominent or projecting jaws. In entomological usage, the prognathous condition is characterized by an occipital foramen near the vertexal margin with mandibles directed anteriad and positioned at the anterior margin of the head. When viewed in lateral aspect, the primary axis of the head is horizontal. Some predaceous insects, such as carabid beetles and earwigs, display the prognathous condition. In other insects, such as cucujid beetles and bethylid wasps, the prognathous position may reveal a solution to problems associated with living in concealed situations such as between bark and wood or similar confined habitats.
In general zoological usage, “opisthognathous” (Greek, opisthos = behind; gnathos = j aw) refers to animals with retreating jaws. In entomological usage, the opisthognathous condition is characterized by posteroventral position of the mouthparts resulting from a deflection of the facial region. The opisthognathous condition is displayed in many fluid-feeding Homoptera, including leafhoppers, whiteflies, and aphids.
SUTURES OF THE HEAD
Head sutures are sometimes used to delimit specific areas of the head, but there are problems. Establishing homology of sutures between families and orders is difficult. From a practical viewpoint, standards have not been developed for naming sutures among insect groups. Some names are based on the areas delimited (e.g., frontoclypeal suture); other sutures are named for the areas in which the suture is found (e.g., coronal suture). Sutures frequently have more than one name (e.g., frontoclypeal suture and epistomal suture are synonymous).
The compound eye is an important landmark on the insect head. An ocular suture surrounds the compound eye and forms an inflection or an internal ridge of the integument (Figs. 3 and 4). The ocular suture is not present in all insects and is difficult to see in some insects unless the head is chemically processed for microscopic examination. When present, the ocular suture probably provides strength and prevents deformation of the compound eye.
A subocular suture extends from the lower margin of the compound eye toward the subgenal suture. In some species the sub-ocular suture (Fig. 4) may extend to the subgenal suture; in other species it may terminate before reaching another landmark. This suture is straight and commonly found in the Hymenoptera, where it may provide additional strength for the head.
POSTERIOR ASPECT OF THE HEAD
The entire posterior surface of the head is termed the postcranium (Fig. 5). The surface may be flat, concave, or convex, depending on the group of insects. The occiput (Latin, back of head) of pterygote insects is the posterior portion of the head between the vertex and cervix (Latin, neck). The occiput is rarely present as a distinct sclerite or clearly demarcated by “benchmark” sutures. When present, the occiput signifies a primitive head segment. In some Diptera the occiput forms the entire posterior surface of the head. In other insects it forms a narrow, horseshoe-shaped sclerite.
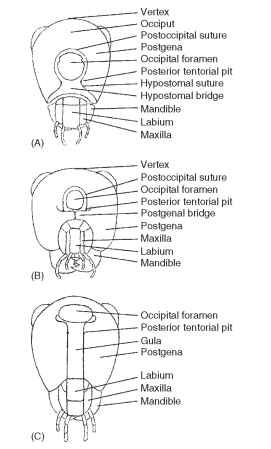
FIGURE 5 Occipital closures: (A) hypostomal bridge, (B) postge-nal bridge, and (C) gula.
The occipital suture (hypostomal suture sensu MacGillivray) is well developed in orthopteroids, but it is not present in many other groups of pterygote insects (Fig. 4) . When present, the occipital suture forms an arched, horseshoe-shaped groove on the back of the head that ends ventrally, anterior to the posterior articulation of each mandible. Internally, the occipital suture develops into a ridge, providing strength for the head.
The postoccipital suture is a landmark on the posterior surface of the head and is typically near the occipital foramen (Fig. 5A and 5B). The postoccipital suture forms a posterior submarginal groove of the head with posterior tentorial pits marking its lower ends on either side of the head. Some morphologists regard this suture as an intersegmental boundary (labium) between the first and second maxillae. Internally, the postoccipital suture forms the postoccipital ridge that serves as an attachment for the dorsal prothoracic and cervical muscles of the head. The absence of the postoccipital suture in pterygote insects is a derived condition.
The postocciput of pterygotes forms the extreme posterior, often U-shaped sclerite that forms the rim of the head behind the postoc-cipital suture. The postocciput is interpreted as a sclerotic remnant of the labial somite in ancestral insects.
In pterygotes such as Orthoptera the occipital foramen and the mouth are not separated. More highly evolved insects have developed sclerotized separations between the mouthparts and the occipital foramen. At least three types of closure have been identified (Fig. 5): the hypostomal bridge, the postgenal bridge, and the gula. An understanding of these structures provides insight into the operation of the head and suggests evolutionary trends in feeding strategies.
The hypostomal bridge is usually developed in adult heads displaying a hypognathous axial orientation. The bridge is formed by medial extension and fusion of hypostomal lobes (hypostoma) (Fig. 5A). The hypostomal bridge is the ground plan condition of closure for the posterior aspect of the head, but it is not restricted to primitive insects. The hypostomal bridge is found in highly developed members of the Heteroptera, Diptera, and Hymenoptera. In Diptera the hypostomal bridge also has been called the pseudogula.
The postgenal bridge is a derived condition from the hypos-tomal ground plan and is developed in adults of higher Diptera and aculeate Hymenoptera. The bridge is characterized by medial extension and fusion of the postgenae, following a union of the hypostoma (Fig. 5B). The posterior tentorial pits retain their placement in the postoccipital suture.
The gula (Latin, gullet; pl., gulae) is developed in some Coleoptera, Neuroptera, and Isoptera. Typically, the gula is developed in heads displaying a prognathous axial orientation and in which posterior ten-torial pits are located anteriad of the occipital foramen (Fig. 5C). The median sclerite (the gula) on the ventral part of a prognathous head apparently forms de novo in the membranous neck region between the lateral extensions of the postocciput. The gula is a derived condition that is found in some but not all prognathous heads.
Endoskeletal Head Framework
Although the hardened integument of the head forms a structurally rigid capsule, this design is insufficient to solve the problems associated with muscle attachment and maintaining structural integrity during chewing. Thus, insects have evolved a tentorium (Latin, tent; pl., tentoria): a complex network of internal, hardened, cuticu-lar struts that serve to reinforce the head. The tentorium forms as an invagination of four apodemal arms from the integument in most pterygote insects. The tentorium strengthens the head for chewing, provides attachment points for muscles, and also supports and protects the brain and foregut.
Anatomically, the tentorium consists of anterior and posterior arms. In most insects, the anterior arms arise from facial inflections located just above the anterior articulations of the mandibles. Externally,
the arms are marked by anterior tentorial pits positioned on the frontoclypeal or subgenal (pleurostomal) suture (Fig. 4) . Internally, the anterior region may form a frontal plate. Posterior arms originate at the ventral ends of the postoccipital inflection. They are marked externally by posterior tentorial pits (Fig. 4). The posterior arms usually unite to form a transverse bridge or corpus tentorii (internally) across the back of the head. Dorsal arms (rami), found in many insects, arise from the anterior arms. They attach to the inner wall of the head near antennal sockets. The dorsal arms are apparently not an invagination of cuticle, because pits do not mark them externally.
Mandible Articulation and Musculature
The hypothetical ancestor of insects is thought to possess a mandible with one point of articulation. Later, insects acquired a second point of articulation. The basis of this assumption comes from a survey of the Hexapoda in that the modern Apterygota have a mono-condylic mandible and the Pterygota have a dicondylic mandible.
The term condyle (Greek, kondylos = knuckle) refers specifically to a knoblike process. For the mandible, the condyle is the point of articulation with the head. On the head itself is an acetabulum (Latin, acetabulum = vinegar cup), a concave surface or cavity for the reception and articulation of the condyle.
The dicondylic mandible is the derived condition and is found in the Lepismatidae and Pterygota. The dicondylic mandible has secondarily acquired an articulation point anterior to the first point in the monocondylic mandible. These attachments form a plane of attachment. In the monocondylic mandible there is no plane of attachment, and the mandibles move forward or rearward when the muscles contract. The two points of articulation create a plane of movement that restricts the direction of mandible movement.
THORAX
The thorax represents the second tagma of the insect body. The thorax evolved early in the phylogenetic history of insects. In most Paleozoic insects the thorax is well developed and differentiated from the head and abdomen, and the three distinct tagmata probably developed during the Devonian.
In terms of insect phylogeny, the thorax of Apterygota is strikingly different in shape compared with the head or abdomen. Of modern apterygotes only the Collembola display taxa in which thoracic tag-matization and segmentation are not obvious.
Apparently, the primary, functional role of the thorax has always been locomotion, since the primary modifications of the thorax have been for locomotion (first walking, and then flight). Modification for locomotion probably developed before other morphological adaptations, such as metamorphosis. Diverse independent and interdependent mechanisms for locomotion have evolved throughout the Insecta, including walking, flight, and jumping. Active participation in flight by insects is unique among invertebrates.
Anatomy of the Thorax
The cervix is the connection between the head (occipital foramen) and the anteriormost part of the thorax (pronotum) (Fig. 2). Typically, the area between the head and pronotum is membranous. The ground plan for the insect cervix contains two cervical sclerites on either side of the head that articulate with an occipital condyle of the head and the prothoracic episternum. Musculature attached to these sclerites increases or decreases the angle between the sclerites, and creates limited mobility of the head.
The thorax of modern insects consists of three segments termed the prothorax, mesothorax, and metathorax. The last two collectively are called the pterothorax (Greek, ptero = wing or feather) because extant insects bear wings on these segments only. The individual dorsal sclerites or terga of the thoracic segments are also known as nota (Greek, notos = back; sing, notum). The nota of Apterygota and many immature insects are similar to the terga of the abdomen with typical secondary segmentation. The nota of each thoracic segment are serially distinguished as the pronotum, mesonotum, and metanotum.
The size and shape of the prothorax are highly variable. The prothorax may be a large plate as in Orthoptera, Hemiptera, and Coleoptera, or reduced in size forming a narrow band between the head and mesothorax as in Hymenoptera. The prothorax is usually separated or free from the mesothorax. The sclerites are separated by a membrane that may be large and conspicuous in more primitive holometabolous insects such as Neuroptera and Coleoptera, or reduced in size in more highly evolved holometabolous insects such as Diptera and Hymenoptera.
The pterothorax includes the thoracic segments immediately pos-teriad of the prothorax. In winged insects the relationship between thoracic segments involved in flight can be complicated. In contrast, the thorax of larval insects and most wingless insects is relatively simple. The mesothorax and metathorax of these insects are separated by membrane. Adult winged insects show a mesothorax and metath-orax that are consolidated (i.e., more or less united) to form a functional unit modified for flight.
The development of the pterothoracic segments varies among winged insects. When both pairs of wings participate equally in flight, the two thoracic segments are about the same size. This condition is seen in Odonata, some Lepidoptera, and some Neuroptera. When one pair of wings is dominant in flight, the corresponding thoracic segment is commensurately larger and modified for flight, whereas the other thoracic segment is reduced in size. This condition is seen in Diptera and Hymenoptera, where the forewing is large and dominant in flight. The reverse condition is seen in the Coleoptera, where the hind wing is large and dominant in flight.
In more closely related insect groups, such as families within an order, that are primitively wingless or in which wings have been secondarily lost in modern or extant species, many modifications to the thorax occur. Many wingless forms can be attributed to environmental factors that promote or maintain flightlessness. For instance, island-dwelling insects are commonly short winged (brachypterous), or wingless, whereas their continental relatives are winged, presumably because for island species, flight increases the likelihood of being carried aloft, moved out to sea, and subsequently lost to the reproductive effort of the population. The anatomical consequences of flightlessness can be predictable; in the Hymenoptera, short wings bring a disproportionate enlargement of the pronotum and reduction in size of the mesonotum and metanotum.
Sutures and Sclerites of Wing-Bearing Segments
The wing-bearing segments of the thorax are subdivided into a myriad of sclerites that are bounded by sutures and membranous areas. These sutures and sclerites are the product of repeated modification of the thorax in response to various demands placed on the insect body by the environment. Similar modifications have occurred independently in many groups of insects; some modifications are unique. Generalizations are difficult to make, given the large number of sutures and sclerites, coupled with the number of insects that there are to consider.
Dorsal Aspect
The nota of the pterothorax are further subdivided into the pres-cutum, scutum, and scutellum; again, serially distinguished as mes-oscutum and mesoscutellum, and metascutum and metascutellum (Fig. 6). Additionally, there are sclerites anterior and posterior to the notum, as discussed shortly.
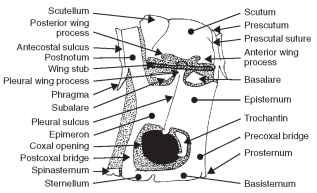
FIGURE 6 Diagram of the pterygote pterothorax.
The prescutum is the anterior portion of the scutum, laterally bearing prealar bridges separated by the prescutal suture from the mesoscutum. The scutum is the largest dorsal sclerite of the notum and is bounded posteriorly by the scutoscutellar suture, which divides the notum into the scutum and scutellum. The scutellum is generally smaller than the scutum. In Heteroptera it is a small triangular sclerite between the bases of the hemelytra. In Coleoptera the scutellum is the small triangular sclerite between the bases of the elytra. In Diptera and Hymenoptera the scutellum is relatively large, forming a subhemispherical sclerite, sometimes projecting posteriad. The posteriormost sclerite of the notum is the postnotum, separated from the notum by secondary segmentation. In some insects there is a postscutellum (metanotal acrotergite) that forms the posteriormost thoracic sclerite of the metanotum, or the posteriormost sclerite of the thorax. In Diptera the postscutellum appears as a transverse bulge below the scutellum.
The acrotergite and postnotum deserve further explanation. Again, the anteriormost sclerite is an acrotergite, the anterior pre-costal part of the notal plate. The postnotum is an intersegmental sclerite associated with the notum of the preceding segment. The postnotum bears the antecosta, a marginal ridge on the inner surface of the notal sclerite corresponding to the primary intersegmental fold. The postnotum also usually bears a pair of internal projecting phragmata. The antecostal suture divides the acrotergite from the antecosta, the internal ridge marking the original intersegmen-tal boundary. Thus, when the antecosta and acrotergite are developed into larger plates and are associated with the notum anterior to them, they are referred to as a postnotum. The final structure associated with the dorsal aspect of the pterothorax is the alinotum (Greek, ala = wing; notos = back; pl., alinota). The alinotum is the wing-bearing sclerite of the pterothorax.
Wing Articulation
The thoracic components necessary for wing movement include the prealar bridge, anterior notal wing process, and posterior notal wing process. The components of the wing itself that articulate with the thoracic components are the humeral and axillary sclerites; they form the part of the wing closest to the body and are not treated in this article.
The prealar bridge is a heavily sclerotized and rigid supporting sclerite between the unsclerotized membrane of the pterothorax and the pleuron; it supports the notum above the thoracic pleura. The prealar bridge is composed of cuticular extensions from the anterior part of the prescutum and antecosta. The anterior notal wing process is the anterior lobe of the lateral margin of the alinotum supporting the first axillary sclerite (Fig. 6). The posterior notal wing process is a posterior lobe of the lateral margin of the alinotum that supports the third axillary sclerite of the wing base (Fig. 6 ).
Lateral Aspect
The pleuron (Greek = side; pl., pleura) is a general term associated with the lateral aspect of the thorax. Adults, nymphs, and active larvae all display extensive sclerotization of the pleural area. Sclerites forming this part of the body wall are derived from the precoxa, sub-coxa, or supracoxal arch of the subcoxa.
PLEURAL REGIONS OF THE THORAX
Apterygota and Immature Plecoptera The anapleurite is the sclerotized area above the coxa (supracoxal area) (Fig. 7). The coxopleurite is a sclerotized plate situated between the coxa and the anapleurite (Fig. 7). It bears the dorsal coxal articulation, the anterior part of which becomes the definitive trochantin. The sternop-leurite, or coxosternite, is the definitive sternal sclerite that includes the areas of the limb bases and is situated beneath the coxa (Fig. 7).
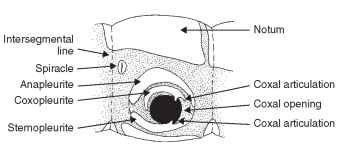
FIGURE 7 Pleural aspect of apterygote thorax.
Pterygota The basalare is a sclerite near the base of the wing and anterior to the pleural wing process (Fig. 8). The basalare serves as a place of insertion for the anterior pleural muscle of the wing. The subalare is posterior to the basalare and the pleural wing process (Fig. 8). It too serves as a place for insertion of the wing’s posterior pleural muscle. The tegula is the anteriormost independent scle-rite associated with the wing base. The tegula is typically scalelike, articulates with the humeral sclerite, and protects the wing base from physical damage. The tegula is absent from Coleoptera and from the metathorax of most orders. The pleural wing process is located at the dorsal end of the pleural ridge and serves as a fulcrum for the movement of the wing (Fig. 8). The parapteron is a small sclerite, articulated on the dorsal extremity of the episternum just below the wings( Fig. 6 ).
The pleural suture is an easily visible landmark on the pterotho-racic pleura (Fig. 8). It extends from the base of the wing to the base of the coxa. The pleural ridge is formed internally by the pleural suture and braces the pleuron above the leg. The episternum is a pleural sclerite anterior to the pleural suture and sometimes adjacent

FIGURE 8 Lateral aspect of the pterygote thorax (Orthoptera: Acrididae).
to the coxa (Fig. 8); the episternum is typically the largest lateral thoracic sclerite between the sternum and the notum. The epimeron is the posterior division of a thoracic pleuron adjacent to the coxa and posterior to the pleural suture (Fig. 8) ; it is typically smaller than the episternum and narrow or triangular. The episternum and the epimeron of many insects have become subdivided into several secondary sclerites bounded by sutures. The simplest condition shows the episternum divided into a dorsal anepisternum and a ventral katepisternum (Fig. 8) . Similarly, the epimeron is divided into an anepimeron and katepimeron. The trochantin is a small sclerite at the base of the insect leg of some insects (Figs. 6 and 8 ). Some workers theorize that the trochantin may have developed into the pleural wall. The trochantin is often fused to the episternum or absent.
The precoxal bridge is anterior to the trochantin, usually continuous with the episternum, frequently united with the basisternum, but also occurs as a distinct sclerite (Fig. 8). The postcoxal bridge is the postcoxal part of the pleuron, often united with the sternum behind the coxa (Fig. 8). The sclerite extends behind the coxa and connects the epimeron with the furcasternum. The meron is a lateral, postarticular basal area of the coxa and is sometimes found disassociated from the coxa and incorporated into the pleuron. The meron is typically large and conspicuous in panorpid and neurop-teran insects. In Diptera the meron forms a separate sclerite in the thoracic pleuron.
Ventral Aspect
The ground plan of the sternum (Greek, sternon = chest; pl., sterna) consists of four sclerites, including an intersternite (spinaster-nite), two laterosternites (coxosternites), and a mediosternite (Fig. 9 ). The mediosternite and the laterosternite meet and join, and the line of union is called the laterosternal sulcus (pleurosternal suture) (Fig. 9).
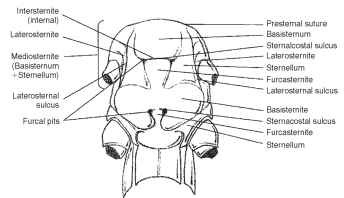
FIGURE 9 Ventral aspect of the thorax (Orthoptera: Acrididae).
Paired furcal pits are found in the laterosternal sulcus (Fig. 9). A transverse sternacostal sulcus bisects the ventral plate and thereby forms an anterior basisternite and posterior furcasternite (Fig. 9) . The basisternite (basisternum) is the primary sclerite of the sternum (Fig. 9). It is positioned anterior to the sternal apophyses or sterna-costal suture and laterally connected with the pleural region of the precoxal bridge. The furcasternite (furcasternum) is a distinct part of the sternum in some insects bearing the furca (Fig. 9). The spin-asternum is a “spine-bearing” intersegmental sclerite of the thoracic venter, associated or united with the preceding sternum. The spin-asternum may become part of the definitive prosternum or mesos-ternum, but not of the metasternum. The sternellum is the second sclerite of the ventral part of each thoracic segment, frequently divided into longitudinal parts that may be widely separated (Figs. 6 and 9).
ABDOMEN
The abdomen is more conspicuously segmented than either the head or the thorax. Superficially, the abdomen is the least specialized of the body tagma, but there are notable exceptions such as the scale insects. The abdomen characteristically lacks appendages except cerci, reproductive organs, and pregenital appendages in adult Apterygota and larval Pterygota.
Ground Plan of the Abdomen
The ground plan abdomen of an adult insect typically consists of 11-12 segments and is less strongly sclerotized than the head or thorax (Fig. 10). Each segment of the abdomen is represented by a sclerotized tergum, sternum, and perhaps a pleurite. Terga are separated from each other and from the adjacent sterna or pleura by a membrane. Spiracles are located in the pleural area. Modification of this ground plan includes the fusion of terga or terga and sterna to form continuous dorsal or ventral shields or a conical tube. Some insects bear a sclerite in the pleural area called a laterotergite. Ventral sclerites are sometimes called laterosternites. The spiracles are often situated in the definitive tergum, sternum, laterotergite, or laterosternite.

FIGURE 10 Abdominal segmentation.
During the embryonic stage of many insects and the postembry-onic stage of primitive insects, 11 abdominal segments are present. In modern insects there is a tendency toward reduction in the number of the abdominal segments, but the primitive number of 11 is maintained during embryogenesis. Variation in abdominal segment number is considerable. If the Apterygota are considered to be indicative of the ground plan for pterygotes, confusion reigns: adult Protura have 12 segments, Collembola have 6. The orthopteran family Acrididae has 11 segments, and a fossil specimen of Zoraptera has a 10-segmented abdomen.
Anamorphosis is present among some primitive ancestral hexa-pods such as the Protura—they emerge from the egg with eight abdominal segments and a terminal telson. Subsequently, three segments are added between the telson and the last abdominal segment with each molt. In contrast, most insects undergo epimorphosis in which the definitive number of segments is present at eclosion. Given the extent of variation in abdominal segmentation, morpholo-gists conventionally discuss the abdomen in terms of pregenital, genital, and postgenital segmentation.
Abdominal Anatomy
Typically, the abdominal terga show secondary segmentation with the posterior part of a segment overlapping the anterior part of the segment behind it (Fig. 10). Such overlap prevents damage or injury to the animal while it moves through the environment, particularly in confined spaces.
The pregenital segments in male insects are numbered 1 through 8; the pregenital segments in female insects are numbered 1 through 7 (Fig. 10). Among the Apterygota, male genitalia in Collembola are positioned between segments 5 and 6 and in Protura between segments 11 and the paraproct. Genital segments of Pterygota include segment 9 in males and segments 8 and 9 in females. Postgenital segments of ptery-gote insects are 10 and 11 in females and 9 and 10 in males.
In general there is little modification of the pregenital sclerites. A notable exception is found in the Odonata. Male Odonata do not have an intromittent organ on segment 9. Instead, the male moves the abdominal apex forward and deposits sperm in a reservoir along the anterior margin of the third abdominal sternum. Other modifications of the pregenital sclerites are not related to sexual behavior. Some of these modifications are glandular.
Modification of the genital sclerites from the ground plan is frequently observed among insects. Adult Pterygota are characterized by a well-developed reproductive system, including organs of copulation and oviposition. This duality of function has resulted in considerable differentiation of associated segments and contributed to difference of opinion regarding homology of genitalic parts. Among pterygote insects the male genitalia are generally positioned on segment 9. The ninth sternum is called a hypandrium (Greek, hypo = beneath; aner = male; Latin, -ium = diminutive) in many insects, including Psocoptera. In Ephemeroptera, the tenth sternum is called a hypandrium. Fusion of segments 9 and 10 in Psocoptera results in a structure called the clunium (Latin, clunais = buttock).
The gonopore (Greek, gone = seed; poros = channel) of the female reproductive system serves as the aperture through which the egg passes during oviposition. The gonopore usually is located on segment 8 or 9. Enlargement of sternum 8 in some female insects is called a subgenital plate.
Modification of postgenital sclerites is frequently observed and seems to be a functional response to adaptations associated with copulation and oviposition. Some modifications include fusion of the tergum, pleuron, and sternum to form a continuous sclerotized ring. The phenomenon is notable in apterygota and pterygote insects.
The eleventh abdominal segment forms the last true somite of the insect body. Frequently, this segment is found in embryonic stages of primitive insects even when it cannot be observed in postemer-gent stages. When the eleventh segment is present, it forms a conical endpiece that bears an anus at the apex, flanked laterally by cerci (Greek, kerkos = tail) (Fig. 10). The dorsal surface of the eleventh segment is called an epiproct (Greek, epi = upon; proktos = anus); the ventrolateral surface is called a paraproct (Greek, para = beside;proktos = anus) (Fig. 10). A longitudinal, medial, membranous area connects the paraprocts ventrally. Primitive groups of extant insects such as Thysanura and Ephemeroptera, and some fossil groups such as Paleodictyoptera, display a conspicuous, long, median filament that apparently projects from the apex of the epiproct. This is called the appendix dorsalis or caudal style. The appendage appears annu-lated and similar in shape to the lateral cerci, but the function of the appendix is unknown. The twelfth abdominal segment is called the periproct in Crustacea, and it forms a telson in some embryonic insects. The periproct appears in adult Protura and naiadal Odonata.
Abdominal Appendages
Presumably, the hypothetical ancestor of the Insecta was a myr-iapod with one pair of appendages for each body segment. Among contemporary insects the head appendages are represented by the antennae, mandibles, and the first and second maxillae. Thorax appendages are represented by legs, whereas the wings are considered to be secondary in origin. In most Apterygota, paired abdominal appendages are apparent. In most true insects embryological appendages are formed and lost before eclosion. The appendages found in embryos apparently represent ancestral conditions that are not expressed in postembryonic stages of modern insects. In modern insects, most pairs of appendages have been lost, and the irregular distribution of the remaining appendages makes a summary evaluation difficult. Abdominal appendages do not resemble the structure of thoracic legs of any insect.
Appendages are common among some entognathous hexapods, and some ancestral forms display unique abdominal appendages. Collembola are highly specialized entognathous Hexapoda. The abdomen of Collembola bear saltatorial appendages, which gives the group its common name of springtail, and a ventral tube, the collo-phore, which is the basis of the ordinal name.
The collophore (Greek, kolla = glue; pherein = to bear) is found on the first abdominal segment of Collembola. The collophore forms a ventromedial tube that is eversible with hydrostatic pressure and is drawn inward with retractor muscles. Some morphologists believe the collophore represents the fusion of paired, lateral appendages of an ancestor. An early explanation of the collophore function noted it was an organ of adhesion. The collophore also is used as a grooming organ in some Collembola. The collophore is connected to secretory glands in the head, and the median longitudinal channel on the venter of the thorax extends from the head to the base of the collophore.
OTHER APPENDAGES
Protura maintain short, cylindrical appendages on each of the first three abdominal segments. Each of these arises from membranous areas between the posterolateral angles of the terga and sterna. The position suggests a pleural origin.
APPENDAGES OF PTERYGOTA The aquatic neuropteran
larva Sialis has long, tapering, six-segmented appendages on each of the first seven body segments. These appendages articulate to pleu-ral coxopodites. Similar appendages are found on the abdomen of some aquatic coleopteran larvae.
The tenth abdominal segment is present in most larval and adult Holometabola. As noted earlier, it is sometimes fused with segment 11. Segment 10 displays paired appendicular processes called pygopodia in Trichoptera, Coleoptera, and Lepidoptera. Pygopods form terminal eversible appendages in some beetle larvae. Pygopodia are bilaterally symmetrical, with eight podia, or feet, per side. Control of the podia is apparent because they are not always everted or inverted. Podia are withdrawn into the segment and have a common or median stalk. Each podium has several rows of equally spaced acanthae that apparently serve as holdfasts. Functionally, the acanthae enable the larvae to attach to and move on different substrates. When the larva walks on a flat substrate, the pygopodia are retracted into the body. When the larva walks on the edge of a leaf, the pygopodia are everted and used as holdfasts.
The larval prolegs of terrestrial Lepidoptera and Symphyta are not well developed, but they are adapted to grasping substrates. These structures are considered to be serially homologous with legs, but they are also referred to by some as adaptive structures with no relation to legs.
The adult pterygote abdomen has appendages that are not generally observed. These appendages are grouped for discussion based on the segments of the abdomen on which they are found.
Pregenital appendages are rare among insects. Adult white-flies have a curious structure on sternum 8 that propels honeydew away from the body. Genitalia are segmental appendages and are treated in the next section. Postgenital appendages include cerci (Latin, circle), which are thought to represent primitive appendages because they are found in the Apterygota (except Protura) and many Pterygota. Cerci originate on abdominal segment 11 in a membranous area between the epiproct and the paraproct (Fig. 10). In insects that have lost segment 11, the cerci appear to originate on segment 10. Cerci occur in all orders among the Hemimetabola except for hemipteroids; among the Holometabola, they are found only in the Mecoptera and Symphyta.
Cerci are highly variable in size and shape and function. They are longer than the body in Thysanura, and in some Orthoptera cerci may be indistinct. Cerci resemble forceps in Japygidae and are annu-lated in Dictyoptera. In Dictyoptera they detect air currents, are sensitive to sound, and may be chemoreceptive. Some Ephemeroptera use cerci to propel themselves through water. Japygidae and Dermaptera probably use cerci to subdue prey. In some groups such as Embioptera and Orthoptera, cerci are sexually dimorphic and may serve a role in copulation.
There are some features on the insect body that appear as appendages but are not. Urogomphi (Greek, oura = tail; gomphos = nail; sing., urogomphus) are fixed or mobile cuticular processes on the apical abdominal segment of some coleopteran larvae. They may or may not be homologous with cerci, or other true appendages.
GENITALIA
The examination of the reproductive anatomy of different insect orders helps to develop an appreciation for the evolutionary trends in the formation of the external genitalia. The male genitalia are derived from the ninth abdominal segment. The female genitalia are derived from the eighth and ninth abdominal segments. In the female, the aperture through which the egg passes is called a gonop-ore. The gonopore serves as a boundary between the external and internal genitalia and is usually independent of the anus. Exceptions include some flies, such as the Tephritidae, where a common lumen termed a cloaca serves for excretion, copulation, and oviposition.
There is usually a single, medially located gonopore. The Der-maptera and Ephemeroptera are ancient groups of hemimetabolous insects. Both orders display a condition in which the lateral oviducts do not combine to form a median oviduct. Instead, the lateral oviducts independently connect with paired gonopores on the conjuctival membrane along the posterior margin of the seventh abdominal segment.
Many female insects with a genitalic opening on the posterior margin of the eighth abdominal segment display an appendicular ovipositor (Fig. 11). The ovipositor is a structure that develops from modified abdominal appendages or segments. It functions in the precise placement of eggs. It is commonly assumed that insects that do not show an ovipositor have ancestors that had an ovipositor. Thus, the structure has been lost during the course of evolutionary adaptation to a particular lifestyle. Female insects with a genitalic opening on the posterior margin of the ninth abdominal segment typically display a rudimentary or suppressed appendicular ovipositor. These insects lack special provisions for egg placement, but sometimes they reveal other abdominal modifications intended to facilitate oviposition.

FIGURE 11 Appendicular ovipositor (Orthoptera: Tettigoniidae).
Female Genitalia
Morphologists often use the Thysanura as a starting point for developing a generalized model to explain the evolution of the external reproductive system of pterygote insects. The thysanuran abdomen has basal sclerotized plates called coxopodites on which styli are attached. These plates are serially homologous along the abdomen, and the pregenital plates are regarded as identical with the genital plates. The plates located on segments 8 and 9 are considered to be genital plates. The styli associated with these segments are called gonapophyses. There are four gonapophyses on segments 8 and 9 (i.e., a pair of styli on each segment). The gonapophyses are medially concave and directed rearward. The basal sclerite is called a gono-coxa, and in some Thysanura it may be fused with the style.
The primitive pterygote with a gonopore on segment 8 has an appendicular ovipositor that consists of three components. A basal apparatus corresponds to the basal plate or primitive gonocoxite of the thysanuran abdominal appendage. The second part is the first valvifers (on the eighth sternum), and second valvifers (on the ninth sternum) are responsible for providing support and points of articulation for the tube through which the egg passes (Fig. 12). Interpolated between the first and second valvifers is a small scle-rite called a gonangulum, which articulates with the second gono-coxite and tergum 9. The gonangulum is present in Odonata and Grylloblatoidea. It apparently is fused with the first valvifer in Dictyoptera and Orthoptera. In the remaining orders these structures are highly variable.
The shaft of the ovipositor consists of two pairs of elongate, closely appressed sclerites called the first and second valvulae (Fig. 12). The first pair of valvulae is positioned on the eighth abdominal sternum. The second pair of valvulae is located on the ninth abdominal sternum and is dorsal in position. Third valvulae are positioned on the posterior end of the second valvifers. These valvulae usually serve as a sheath for the shaft of the ovipositor (Figs. 11 and 12).
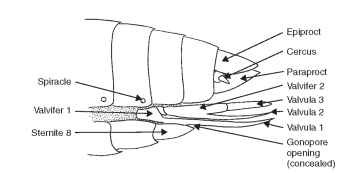
FIGURE 12 Female genitalia (diagrammatic), based on orthop-teran female.
Male Genitalia
The primary function of the male genitalia in insects is insemination of the female. Methods of achieving insemination that involve special functions of the external genitalia include clasping and holding the female, retaining the connection with the female gonopore, the construction of spermatophores, and the deposition of spermato-phores or semen into the female genital tract; in some insects the injection of semen takes place directly into the female body (traumatic insemination of some Hemiptera). Other functions of the male genitalia include excretion and various sensory functions.
The genitalia of male insects exhibit such an enormous variety of shapes and constituent parts, often further complicated by structural rotation or inversion of all or some of the parts, that determination of a ground plan is virtually impossible. Examination of ancient orders shows highly variable and specialized conditions. In general, the coxites of the eighth segment in most apterygotes are reduced and without gonapo-physes, and they are absent altogether in the Pterygota. Thus, the male external genitalia are derived from the ninth abdominal coxites.
Again, the Thysanura have genitalia that closely resemble that of the pterygote orders: a median intromittent organ or phallus, and paired lateral accessories (the periphallus of Snodgrass). The phallus is a conical, tubular structure of variable complexity (Fig. 13) . Primitive insects may not display differentiated parts, and the entire structure may be long, sclerotized, and tapering apicad. In a ground plan condition for pterygote insects, there is a sclerotized basal portion termed the phallobase and a distal sclerotized portion called the aedeagus (Fig. 13). The phallobase in insects is characterized by highly variable development: sometimes sclerotized and supporting the aedeagus, sometimes forming a sheath for the aedeagus. The phallobase often contains an apodeme, which may provide support or a point for muscle attachment. The phallobase and aedeagus are joined by a membranous phallotheca (Fig. 13 ) . The external walls of the phallobase and aedeagus are called the ectophallus (Fig. 13). The gonopore is positioned at the apex of the ejaculatory duct and is concealed within the phallobase. The gonopore is connected to the apex of the aedeagus via a membranous tube called the endophallus (Fig. 13). In some insects the endophallus may be everted through the aedeagus. The circular aperture at the apex of the aedeagus is called the phallotreme (Fig. 13). In some insects the endophal-lus and the gonopore may be everted through the phallotreme and into the female’s bursa copulatrix. Genital lobes referred to as phal-lomeres form at the sides of the gonopore in the ontogeny of some insects. Usually the phallomeres unite to form the phallus.

FIGURE 13 Male genitalia (diagrammatic).
