Polymeric saccharides can have 10,000 or more sugar units, four or five different sugars, and a branched structure. The diversity that can be envisioned is huge and much of it is actually observed. Classification of polysaccha-rides differentiates homopolymers (a single sugar type) and heteropolymers (two or more sugar types) with subtypes reflecting linear or branched structures within each group. The physical and biological properties of each polymer depend on the architecture of the molecule as well as the enzymatic machinery available to interact with it. Thus, cellulose, a linear j-1-4 glucose polymer is a stable, highly organized polymer, indigestible by man, whereas starch, its a-linked counterpart is a major food source for all mammalian species.
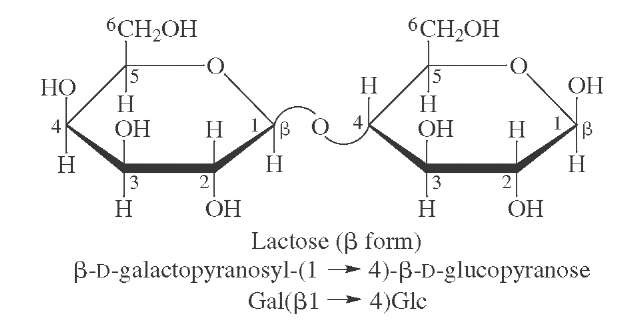
FIGURE 19 Structure of lactose, the major sugar present in milk.
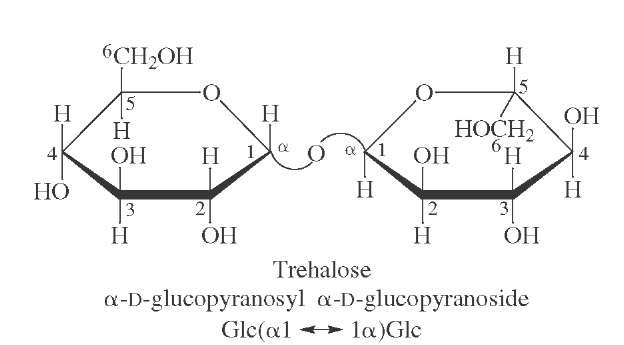
FIGURE 20 Structure of trehalose, the predominant sugar present in the blood of insects.
A. Homopolysaccharides
The major homopolysaccharides are cellulose, chitin, starches (amylose and amylopectin), glycogen, and xylans.
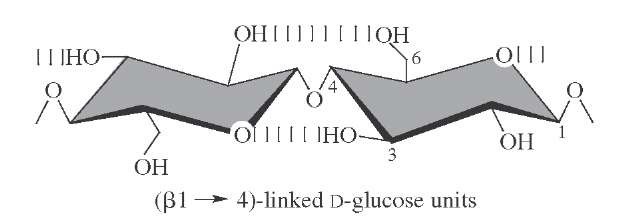
Cellulose, a linear glucose polymer linked j-1-4 is the predominant natural product in the biosphere. The all-equatorial structure allows for extensive hydrogen bonding, whereas the axial, somewhat hydrophobic faces favor a nonaqueous environment. The resulting aggregates have a highly ordered, quasi-crytalline arrangement (Fig. 21); hence, the unusual stability exhibited by the molecule, exemplified in trees and wooden artifacts. The broad distribution and low cost of cellulosehas madeit a major starting material for industrial development while the unmodi-fiedpolymerremains thebasis forwood,paper, andcotton.
Chitin, identical in linkage to cellulose but composed of N-acetylglucosamine instead of glucose, is the major structural component of insect and crustacean exoskele-tons as well as a cell wall component of molds and fungi. The structural comments regarding cellulose also apply generally to chitin, especially in terms of stability. Less industrial development has been done with this polymer, in part because shrimp shells may present a more difficult starting material than trees.
Amylose and amylopectin are closely related, a-1-4-linked glucose polymers. They are major constituents of starches and hence key nutrients worldwide. Amylose is a linear chain with up to a few hundred glucose units. Amylopectin has the same backbone chain but a-1-6 branches approximately every 20 glucose units. The branches have the same linkages as the main chain. This ramified structure allows for efficient packing in cells.
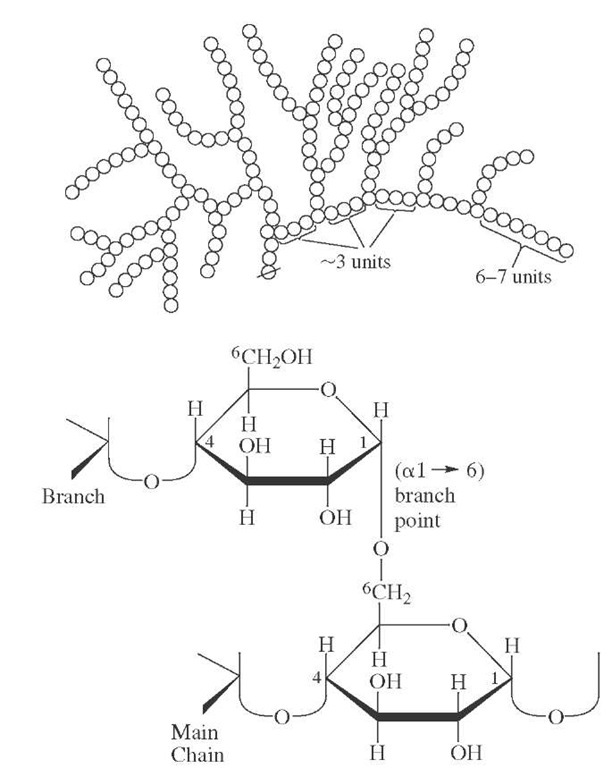
Glycogen (Fig. 22), present in all higher animals, is closely related to amylopectin in that it has the same fundamental structure of a linear glucose chain with branches, and the same linkages. In this case, however, branches occur about every seventh residue, yielding a highly rebranched, tree-like envelope. This is essential for both packing in cells and for the rapid degradation of the polymer to provide critical metabolic intermediates. Glycogen serves as a primary energy reservoir in muscle and as a source of circulating glucose in the liver. It is of interest that the biosynthesis of glycogen initiates on a core protein (glycogenin), which may be cleaved from the polysaccha-ride subsequent to polymerization.
FIGURE 21 The repeating unit of cellulose showing hydrogen bond interactions. The extended structure allows chains to stack via the relatively hydrophobic axial faces of the pyranose rings.
Xylans are a group of polymers based on a structure analogous to that of cellulose wherein xylose is the repeating unit. The simplest representative contains only D-xylose with j-1-4 linkages and is a common component of plant walls. Several heteropolysaccharides utilize the xylan backbone and have various other saccharides as branches. Xylans are often associated with cellulose in plant cell walls.
B. Heteropolysaccharides
The diversity of heteropolysaccharides is enormous. Distributed throughout the animal and plant kingdom, this class of macromolecules rivals proteins in diversity. Initially thought to function only as structural components, it is now realized that specific sequences may have information content and can serve as recognition or signaling elements. In addition, modifications of saccharides already incorporated into a polymeric chain (sulfation, for example) add an additional level of complexity. The ability of these materials to function as other than strictly physical components necessitates, in many cases, that the saccharide chain have a defined three-dimensional conformation. Much work has been done to model the three-dimensional structure ofproteins and many protein structures have been determined by X-ray crystallographic analysis. Much less has been done with complex saccharides in terms of either molecular dynamics or conformational analysis of larger structures. The following discussion is intended to be representative only.
FIGURE 22 Schematic structure for glycogen and details of a typical branch point. The same linkages are present in amylopectin, which has less frequent branches.
In addition to cellulose and xylan, plant cell walls contain a variety of complex heteropolysaccharides including other glucans. Detailed structures have only been determined for a limited number of these structures and their interactions, possibly covalent, with other plant components have rarely been determined in detail. Commercially important are pectins, polygalcturonides, which are key components of jellies and related uronides (gums) used as thickening agents in a broad variety of applications ranging from ice cream manufacture to the production of printing ink. Agars are galactans that are sulfated and also contain 3,6-anhydro-L-galactose, which have gelation properties. They have broad application in microbiology.
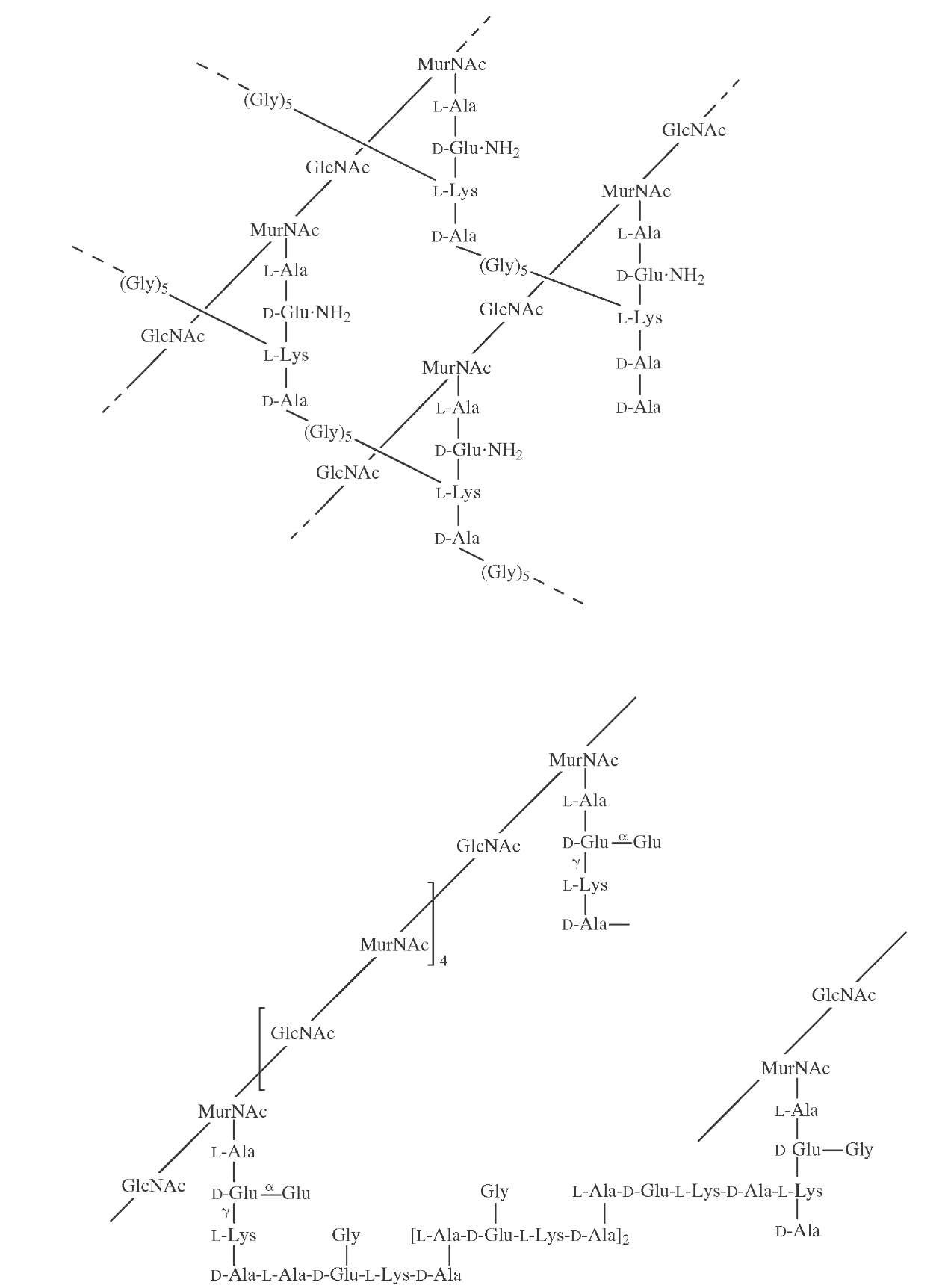
Bacteria likewise produce a wide variety of complex, cell-surface polysaccharides. Strains of Streptococcus pneumoniae, the causative agent of bacterial pneumonia, are characterized by a capsular polysaccharide—more than 100 different types are known. Since the capsular material is immunogenic and protective, polyvalent vaccines have been developed that utilize the capsular polysaccha-rides from common strains. Similarly, organisms responsible for bacterial meningitis have a characteristic polysac-charide capsule that is also immunogenic and protective. Conversely, several bacteria have, as an exterior capsule, saccharides sufficiently similar to those produced in their animal host so as to serve as a mechanism for avoidance of host immune responses. Many gram-positive bacteria have, as essential cell wall components, a complex saccha-ride structure that contains muramic acid (a condensation product of N-acetylglucosamine and pyruvate) and other amino sugars and is cross-linked by a peptide (Fig. 23).
A widely distributed heteropolysaccharide, hyaluronic acid, has both commerical and biological importance. This molecule, a repeating structure of D-glucuronic acid and N-acetylglucosamine with j-1-3 and j-1-4 linkages, is found in bacterial and animal sources, and it is one of the few complex saccharides not covalently linked to protein (Fig. 24). Molecular weights vary depending on source but often exceed two million. The polyanionic nature of the molecule leads to a relatively extended solution conformation. This coupled with the highly hydrophilic chemistry results in solutions with very high viscosity, an important physical property. This is utilized in treatment of osteoathritis of the knee (an injectable) and in eye surgery as a viscoelastic. In addition, cell surface receptors have been identified that recognize the saccharides in hyaluronate, and interaction with specific proteins is responsible for the aggregate properties of connective tissue proteoglycans (see below). This diversity illustrates that a single polysaccharide may have both informational and physical roles in nature.
FIGURE 23 Typical bacterial cell wall structures (peptidoglycans).