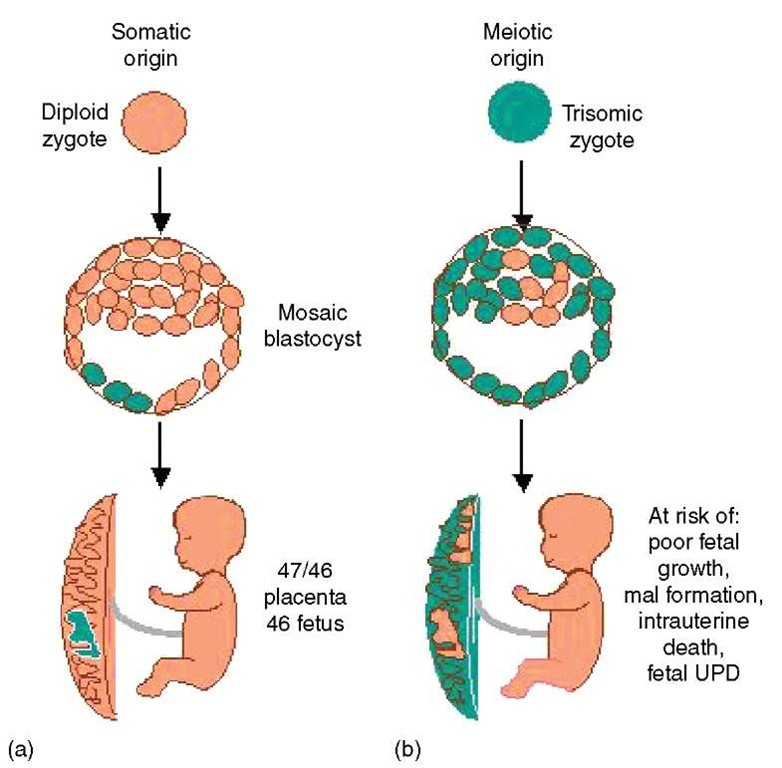
1. Mosaicism-overview
Chromosome mosaicism, the existence of two cell lines with differing chromosomal constitutions derived from a single fertilization, may be observed in amniotic fluid (AF) or chorionic villus (CVS) samples at prenatal diagnosis or in blood or skin samples of individuals referred for a variety of medical conditions. The diagnosis of mosaicism, particularly when ascertained prenatally, presents one of the most problematic genetic counseling situations as it is impossible to fully assess the level and distribution of the abnormal cells or to predict whether outcome of the pregnancy will be normal or not. In cases of mosaicism ascertained postnatally, the abnormal cells may be diagnostic for a specific syndrome, for example, the finding of mosaic tetrasomy 12p in a child with profound mental retardation and other features of Pallister-Killian syndrome (Schinzel, 1991), but can also be inconsequential, for example, a low level of 45,X cells in a woman experiencing recurrent miscarriage (Horsman et al., 1987). The interpretation of a mosaic finding thus needs to be carefully considered in terms of the patient phenotype, the type and origin of the abnormality, and the extent of abnormal cells.
To appreciate the effects of mosaicism, it is first important to consider that all human beings are very likely mosaics. The replication of the cellular genome, while reasonably accurate, is not foolproof and mutations arise and chromosomes segregate improperly with some low probability at each mitotic cell division. How low? That depends on the cell type and the type of abnormality. Studies of unused human embryos from in vitro fertilization (IVF) procedures have indicated that at least 70% are chromosomally abnormal, many of which may show mosaicism with normal diploid cells (Gianaroli et al., 2001; Magli et al., 2001; Ruangvutilert et al., 2000; Wells and Delhanty, 2000). This Figure may well be close to 100% if all cells and all chromosomes could be examined at once. There is presumably a high rate of chromosome missegregation, as well as misdivision of whole haploid complements in the first few postzygotic cell divisions (at least under IVF conditions). Very high rates of chromosome aneuploidy have also been found in normal neurons of the developing and mature mouse brain (Kaushal et al., 2003) and tetraploid cells are common in placental trophoblast of many mammals (Hoffman and Wooding, 1993), suggesting that the production of chromosomally “abnormal” cells may be part of the normal programmed development of some cell types. Aneuploid cells are also found at increasing frequency in cultured lymphocytes as individuals age. The X chromosome seems particularly susceptible to nondisjunction and, on average, about 2-3% of cultured lymphocytes in young women (Fitzgerald and McEwan, 1977; Horsman et al., 1987; Nowinski et al., 1990) and 22% in female centenarians (Bukvic et al., 2001) exhibit X chromosome aneuploidy.
Most embryos with a high percentage of abnormal cells probably do not survive implantation or are aborted in early pregnancy, but it is not known how often low-level mosaics are rescued by the plasticity of early development and go on to produce term births. Mosaicism is detected in 1-2% of CVS and 0.1% of AF samples, mostly involving a trisomic (47 chromosomes) cell line. The lower rate of mosaicism in AF samples reflects both the fact that some abnormal pregnancies will be lost between the time of CVS (8-12 weeks gestation) and amniocentesis (15-20 weeks gestation) and that mosaicism is more commonly found in the placenta as compared to the fetus. However, the true frequency of fetal mosaicism is presumably higher, as AF and fetal blood analysis have been shown in several instances to fail to detect a trisomy that is later found in the fetus or newborn (Bruyere et al., 1999; Desilets et al., 1996; Hammer et al., 1991; Opstal et al., 1998). Chromosomal mosaicism has been observed in as many as 5-15% of placentae examined from healthy term pregnancies when multiple placental sites were examined (Artan et al., 1995). While low levels of trisomic cells in the placenta probably have little impact (and may even be normal), high levels may impair placental function and thus impede fetal growth.
Pregnancy outcome in the case of mosaicism is often associated with how the mosaicism arose. Mosaicism may originate through gain/loss of a chromosome in a normal diploid conceptus (somatic origin) or the abnormal cell line may be present at conception (meiotic origin), but the error “corrects” itself by loss of the supernumerary chromosome during development (Figure 1). Not surprisingly, when the conceptus originates from an abnormal zygote, there tends to be higher levels of trisomy in the placenta, higher risk for fetal mosaicism, and the pregnancy is at higher risk of fetal growth restriction, malformation, and intrauterine or neonatal death (Robinson et al., 1997). Uniparental disomy (see Article 19, Uniparental disomy, Volume 1) (both copies of a chromosome pair originating from the same parent) of the normal cell line can also occur in such cases, which may have a significant phenotypic effect when imprinted genes are located along the involved chromosome. Nonetheless, in many cases, there is a selective advantage of the normal cell line, thus making it possible for a nonmosaic diploid fetus to result from an abnormal conception. For example, trisomy 16 mosaics virtually always derive from a trisomic zygote, but it has been inferred that it is possible to form a baby with entirely (or predominantly) normal cells from only a single diploid cell from the inner cell mass of the blastocyst (Lau et al., 1997; Robinson et al., 2002). While pregnancies with prenatally diagnosed trisomy 16 mosaicism are at increased risk of complications (poor growth, maternal hypertension, and fetal malformations) due to placental trisomy or low-level trisomy in the fetus, it is truly remarkable how often they proceed successfully. In fact, most cases of mosaicism, even when detected in AF cultures, will have a good prognosis (see e.g., Hsu et al., 1997; Wallerstein et al., 2000).
Figure 1 Origin of trisomy mosaicism may be somatic (a) or meiotic (b)
On the flip side, however, is the concern that there may be long-term consequences of mosaicism not apparent at birth. A number of malignancies are associated with chromosomally abnormal cells (see Article 14, Acquired chromosome abnormalities: the cytogenetics of cancer, Volume 1). Some examples include hepatoblastoma with trisomy 20, various hematological malignancies with trisomy 8 (Maserati et al., 2002), certain leukemias with trisomy 21, and gonadoblastoma with 45,X/46,XY mosaicism. While some of these trisomies arise by somatic mis-segregation in the affected tissue, some may be associated with undiagnosed low-level mosaicism already present at birth. For example, a case of erythroleukemia was diagnosed in a 16-month-old girl with normal development, in which the tri-somy 21 cell line found in blood exhibited two different maternal alleles and was thus determined to be of meiotic origin (Minelli et al., 2001). Trisomy 8 in cases of myelodysplasia and acute leukemia can be found in other tissues in 15-20% of cases, suggesting an early embryonic origin (Maserati et al., 2002).
Malignancies are not the only impact of mosaicism. Skin pigmentation anomalies, such as hypomelanosis of Ito, and asymmetric growth are the clinical features that immediately raise the question of possible chromosomal mosaicism. Rheumatoid arthritis, osteoarthritis, and other inflammatory joint diseases have been associated with trisomy 7 mosaicism in the affected joints (Kinne et al., 2001), and trisomy 21 has been found at increased frequency in the peripheral lymphocytes of individuals with Alzheimer disease (Geller and Potter, 1999). The increase of chromosomally abnormal cells with age may also contribute to the “decay” of the body in ways we do not yet understand.
Although, distinct from mosaicism, chromosomally distinct cells may also be found in an individual because of chimerism or microchimerism. In this case, the cells arise not from a single conceptus but from two separate individuals. True chimeras may result from the fusion of two distinct embryos early in development, but proven examples of this are very few. Some cases of 46,XX/46,XY mosaicism have probably been falsely assumed to be chimeras, when in fact they may result from two chromosome loss events from a 47,XXY conceptus (Niu et al., 2002). Microchimerism can occur in twin pregnancies, whereby cells from one twin can populate the haematopoietic stem cells of another twin through “twin to twin transfusion.” Furthermore, there appears to be a normal exchange of cells between fetus and mother and vice versa during pregnancy that can persist throughout the life span of the recipient individual (Bianchi, 2000). The pregnancy need not go to term, and as many as 500 000 fetal nucleated cells are transfused following elective first-trimester termination of pregnancy. Some investigators have hypothesized that the presence of foreign white blood cells might help to explain certain autoimmune diseases that tend to be more common in women after the age of 40 (Bianchi, 2000; Nelson, 2003). There is also evidence for the involvement of maternal cells in the etiology of neonatal or juvenile autoimmune disorders (Stevens et al., 2003).
Clearly, chromosomal mosaicism and microchimerism play important roles in human disease, which are likely to be appreciated more as clinicians and researchers become more aware of their possible impact. However, tracking these rare cells throughout the body can be a real challenge for researchers. The first step in considering the role of low levels of abnormal cells is to rationally consider both the possibility that they may play a role in disease and also that they may not. Unbiased ascertainment of mosaic cases and long-term follow-up will be key to accurately evaluate these possibilities.