Size and sex
The Bernissart discoveries are notable for comprising two types of Iguanodon. One (Iguanodon bernissartensis – quite literally ‘the Iguanodon that lived in Bernissart’) is large and robustly built, and represented by more than 35 skeletons; the other (Iguanodon atherfieldensis, formerly called I. mantelli – literally ‘Mantell’s Iguanodon’) is smaller and more delicately built (approximately 6 metres in length) and represented by only two skeletons.
These specimens were regarded as distinct species until they were reassessed in the 1920s, by Francis Baron Nopcsa, a nobleman from Transylvania and a palaeontologist. The discovery of two quite similar types of dinosaur that evidently lived in the same place, at the same time, prompted him to ask the simple and yet obvious question: are they males and females of the same species? Nopcsa attempted to determine sexual differences in a number of fossil species. In the case of the Iguanodon from Bernissart he concluded that the smaller and rarer species was the male and the larger and more numerous species was the female. He observed, perfectly reasonably, that it is often the case that female reptiles are larger than males. The biological reason for this is that females often have to grow large numbers of thick-shelled eggs; these drain considerable resources from the body before they are laid.
While this seems quite a reasonable supposition, it is in fact very difficult to prove scientifically. Apart from size, which is surprisingly variable among reptiles as a whole and not nearly as consistent a feature as Nopcsa would have had us believe, the features used to distinguish the sexes among living reptiles are most commonly found in the soft anatomy of the sex organs themselves, coloration of the skin, or behaviour. This is particularly unfortunate because only very rarely do fossils ever preserve such features.
The most valuable evidence would be the discovery of soft anatomical fossils of the sexual organs of Iguanodon – unfortunately, this is an extremely unlikely event. And, since we can never know their true biology and behaviour, we have to be a little cautious and also realistic. For the present, it is safer to record the differences (we may have our own suspicions, perhaps), but simply leave it at that.
A careful study of the more abundant large Iguanodon from Bernissart revealed that a few were smaller than the average.
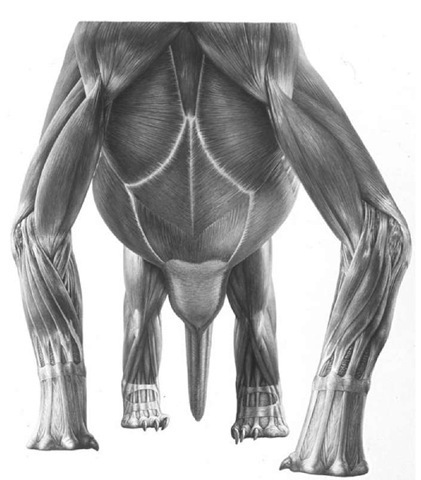
Measuring the proportions of each of these skeletons revealed an unexpected growth change. Smaller, presumably immature specimens had shorter arms than would have been expected. The comparatively short-armed juveniles may well have been more adept bipedal runners, but as large adult size and stature was achieved they may have become progressively more accustomed to moving around on all fours. This also fits with the observation of an intersternal ossification in only larger, presumably adult, individuals, which spent more of their time on all fours compared to smaller, younger individuals.
Soft tissues
Soft tissues of fossil creatures are preserved only rarely, and under exceptional preservational conditions, so palaeontologists have developed techniques to decipher clues concerning this type of biology of dinosaurs both directly and indirectly.
Louis Dollo reported small patches of skin impression on parts of the skeletons of Iguanodon. A number of the skeletons from Bernissart are shown in a classic ‘death pose’ with the powerful neck muscles contracted, during rigor mortis, pulling the neck into a sharp curve and turning the head upward and backward. That this pose has been maintained during the time between death and eventual burial implies that the carcass of the animal had stiffened and dried out. Under such conditions, its tough, parchment-like skin would have formed a rigid surface against which the finegrained muds would have moulded themselves during burial. Provided that the entombing sediment compacted sufficiently to retain their shape, prior to the inevitable rotting and disappearance of the dinosaur’s organic tissues, then (as with simple clay moulds) an impression of the texture of the skin surface would have been preserved in the sediment.
In the case of Iguanodon the texture of the skin impression that was preserved confirmed expectations: it shows a finely scaled, flexible covering, very similar in appearance to that seen on the skin of modern lizards (Figure 23). Clearly, the disappearance of the original tissue means that any traces of skin pigments have long vanished.
23. Iguanodon skin impression
In addition to the detailed work that has to be done simply to describe the bones of the dinosaur’s skeleton, it is also possible to focus on certain parts of the body, notably the hips, shoulders, and head, for clues concerning the arrangement of its muscles. The reason for this is that at the places where muscles and tendons attach to the surface of bones, tell-tale surface markings such as elevated ridges of bone or distinctively pitted muscle scars often form. Skeletal bone is a surprisingly plastic material. Bones must change shape as the body grows, or if it has to repair itself following trauma such as a fracture. What may be less obvious is that even when the body is full grown, its bones continue to be remodelled in response to ever-changing patterns of stress and strain. For example, an individual taking up a course of weight-training will deposit extra skeletal bone in order to cope with the increased load, especially if this training regime is continued over time.
In particular areas of the body, where large muscles exert forces on the skeleton, the scarring on bones can be quite distinctive, even in fossils; this creates a crude map that allows some of the original musculature to be reconstructed (Figure 24). Such reconstructions are based on the known muscular arrangements seen in related living animals, tempered by allowances for the anatomical differences or novelties seen in the fossil animal that is being investigated.
Although far from scientifically ideal, an example of this kind of approach when trying to understand the musculature of Iguanodon is to use as a starting point information from two of the nearest living relatives of dinosaurs: birds and crocodiles. Clearly neither of these types of animal represent, at all accurately, the anatomy of Iguanodon: birds are highly modified for flight, have no teeth, have a minuscule tail and unusually modified hips and leg muscles; crocodiles, though more conventionally reptilian in shape, are highly specialized as aquatic predators. Despite these real problems, they provide a general framework or template – termed the ‘extant phylogenetic bracket’, or EPB – for reconstruction that can be supplemented by the finer details of the anatomy of Iguanodon.
24. Dinosaur muscle reconstruction
The latter includes the general evidence from the overall physical design (shape and arrangement of the bones) of the skeleton or skull, and the influence that these would have on the distribution and functioning of the muscles. Such reconstructions also need to account for such factors as the proposed method of locomotion. For example, the details of the joints between the limb bones, a consideration of the simple mechanics associated with the positioning and range of movement of the limbs that was possible at each limb joint; and, in some cases, the real evidence left behind by dinosaurs in the form of fossilized tracks that indicate how they really did move around when alive.
The extant phylogenetic bracket (EPB)
By creating a phylogenetic tree of the nearest relatives of dinosaurs, it is clear that crocodiles evolved before dinosaurs appeared and that birds evolved after the earliest dinosaurs. Dinosaurs are therefore sandwiched evolutionarily between living crocodiles and living birds.
Anatomical features shared by both living birds and crocodiles should also be present in dinosaurs because they are quite literally ‘bracketed’ by these living creatures. Sometimes this type of approach can help to deduce biological features among extinct groups even when there is no clear physical evidence for such features. However, given how specialized creatures such as dinosaurs can be, when compared to living crocodiles and birds, this approach must be used cautiously.
While examining many bony fragments of Iguanodon in the collections of the Natural History Museum in London, an unusual specimen caught my eye. It consisted of the battered remains of a large, partial skull.
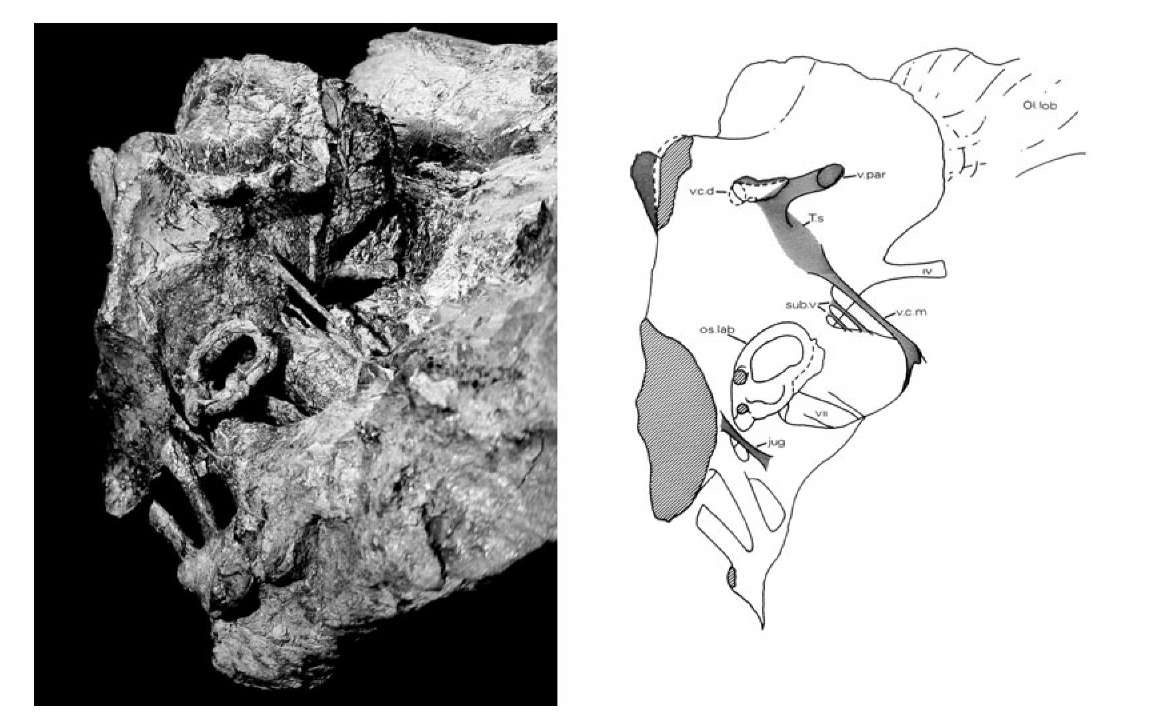
25. Left: oblique view of the natural cast of the brain cavity of Iguanodon. Right: line drawing of the brain cavity showing ear structures, nerves, blood vessels and olfactory lobes.
A few teeth exposed in its upper jaw betrayed that it was indeed Iguanodon, but beyond that it seemed useless anatomically. For interest’s sake, I decided to cut the specimen in half to see if any of its internal anatomy was better preserved. What was revealed proved to be unexpectedly interesting and exciting. Although the bones were battered and eroded, it was clear that this skull had been buried in soft, silty mud that had seeped into all the spaces. The mud had hardened (lithified) to a concrete-like consistency over millions of years. The lithification process was so complete that the mudstone had become impermeable so that ground-water containing minerals was unable to seep through the rock and mineralize the skull bones; as a result the bones were relatively soft and crumbly.
This peculiar preservation offered an unusual opportunity to explore skull anatomy. Careful removal of the crumbly skull bones (rather than the hard mudstone matrix) revealed the shape of the internal spaces in the skull as a natural mudstone cast (Figure 25). It included the cavity where the brain had lain, the passages for the inner ear, and many of the blood vessels and nerve tracts that led to and from the brain cavity. Given that this particular animal had died approximately 130 million years ago, it does seem remarkable that it should prove possible to reconstruct so much of its soft anatomy.
Iguanodon and dietary adaptation
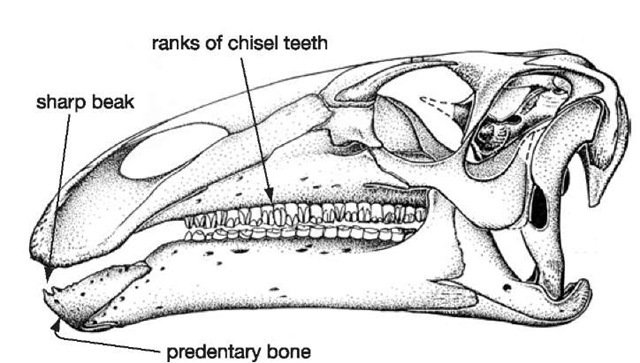
The first recognizable fossils of Iguanodon were teeth, whose telltale features showed that it was a herbivorous animal; they were chisel-shaped to be able to slice and crush plants in the mouth before they were swallowed.
The need to cut and crush plant food hints at some important considerations concerning the diets of extinct creatures and some of the clues that their skeletons may contain.
Iguanodon’s brain
The structure of the brain cavity shows large olfactory lobes at the front, suggesting that Iguanodon had a well-developed sense of smell. Large optic nerves passed through the braincase in the direction of the big eye sockets, apparently confirming that these animals had good vision. The large cerebral lobes indicate a well coordinated and active animal. The inner ear cast shows the looped semicircular canals that provided the animal’s sense of balance, and a finger-like structure that was part of its hearing system. Beneath the brain cavity hangs a pod-like structure that housed the pituitary gland, which was responsible for regulating its hormone functions. Down either side of the cast are seen a series of large tubes, which represent the passages through the original braincase wall (chipped away here of course) for the twelve cranial nerves. Other smaller pipes and tubes passing through the braincase wall are also preserved, and these hint at the distribution of a set of blood vessels that carried blood into the floor of the brain from the heart (via the carotid artery) and, of course, drained the blood away from the brain through the large lateral head veins that lead back down the neck.
Carnivores have a diet largely comprising meat. From a biochemical and nutritional perspective, a diet of meat is one of the simplest and most obvious of options for any creature. Most of the other creatures in the world are made of roughly similar chemicals as the carnivores that eat them. Their flesh is therefore a ready and rapidly assimilated source of food, provided the prey can be caught, sliced into chunks in the mouth using simple knife-like teeth (or even swallowed whole), and then quickly digested in the stomach.
This whole process has the potential to be relatively quick and biochemically very efficient in that little is likely to be wasted.
Herbivores face a rather more challenging problem. Plants are neither particularly nutritious nor readily assimilable when compared to animal flesh. Plants are primarily built from large quantities of cellulose, a material that gives them strength and rigidity. The crucial, and extremely awkward, point about this unique chemical, so far as animals are concerned, is that it is completely indigestible: there is simply nothing in the armoury of chemicals in our guts that can actually dissolve cellulose. As a result, the cellulose portion of plants passes straight through animals’ guts as what we call roughage. So, how do herbivores survive on what appears to be such an unpromising diet?
Plant-eaters have successfully adapted to this diet because they exhibit a number of characteristic features. They have a good set of teeth with hard-wearing, durable, complex, and rough grinding surfaces, and powerful jaws and muscles that can be used to grind up plant tissues between the teeth to release the nutritionally usable ‘cell sap’ that is enclosed within plant cell walls. Herbivores eat large quantities of plant food in order to be able to extract sufficient nutrients from such comparatively nutrient-poor material. As a result, herbivores tend to have barrel-shaped bodies that accommodate large and complicated guts, which are necessary to store the large volumes of plants that they have to eat and allow sufficient time for digestion to take place. Herbivores’ large guts house dense populations of microbes that live within special chambers or pouches in the gut wall; our topic is a tiny vestige of such a chamber, and hints at herbivory in our primate ancestry. This symbiosis allows herbivorous animals to provide a warm, sheltered environment and constant supplies of food for the microbes; in their turn, the microbes have the ability to synthesize cellulase, an enzyme that digests cellulose and converts it into sugars that can then be absorbed by the host animal.
By most standards, Iguanodon (11 metres long and weighing about 3-4 tonnes) was a large herbivorous animal, and would have consumed plants in large quantities. Given this background information, questions about precisely how Iguanodon fed and assimilated its food can be explored in detail.
One persistent theory concerning its method of feeding was its suggested use of a long tongue to pull vegetation into the mouth. This began with Gideon Mantell, who described one of the first, nearly complete lower jaws of Iguanodon. The new fossil included some tell-tale teeth, so the ownership could not be doubted, and it had a toothless, spout-shaped front end. Mantell speculated that the spout shape allowed a long tongue to slide in and out of the mouth, rather like a giraffe’s does. Mantell could not have known that the tip of the newly discovered lower jaw was incomplete and was capped by a predentary bone that filled in the ‘spout’.
It is very curious to note that in the 1920s Louis Dollo provided further support for Mantell’s conjecture. Dollo described a special opening in the predentary at the tip of the lower jaw; this formed a tunnel passing straight through the predentary bone that allowed a long, thin, muscular tongue to be projected outward to grasp vegetation and draw it into the mouth. Large bones (ceratobranchials) that had been found lying between the jaws of Iguanodon were suggested to act as the attachment for the muscles that would have operated this type of tongue. Such a structure fitted neatly with Dollo’s concept of Iguanodon as a high arboreal browser with a giraffe-like long, grasping tongue.
26. Iguanodon skull
Careful re-examination of the lower jaws of a number of Iguanodon skulls from Bernissart failed to reveal Dollo’s predentary tunnel. The predentary has a sharp upper edge that supported a turtle-like horny beak. The predentary, and its beak, bit against the similarly toothless beak-covered premaxillae at the tip of the upper jaw, and this arrangement allowed these dinosaurs to very effectively crop the plants upon which they were feeding. The advantage of the horny beak was that it would have grown continuously (unlike teeth, which gradually wear away) no matter how tough and abrasive the plants that were being cropped. The ceratobranchial bones still require some explanation. In this instance, they would have been used to anchor the muscles that moved the tongue around the mouth to reposition the food as it was being chewed and for pushing the food back into the throat when it was ready to be swallowed. This is exactly the same role that is performed by the ceratobranchial bones in the floor of the human mouth.
How Iguanodon chewed its food
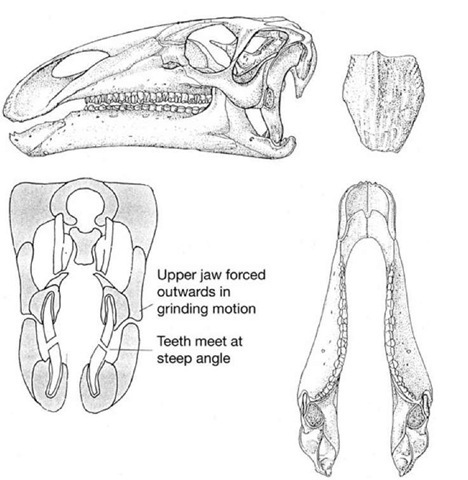
Apart from the horny beak that was able to nip off plants at the front end of the mouth, the sides of the jaws are lined with a formidable, nearly parallel array of chisel-like teeth that form irregularly edged blades (Figure 26). Each working tooth slots neatly against its neighbours in a rank-and-file arrangement, and beneath the working teeth are replacement crowns that will slot into place as the working teeth are worn away, forming what is in effect a ‘magazine’, or battery, of teeth. This continuous replacement pattern is normal for reptiles in general. What is unusual, even by reptile standards, is that the working and replacement teeth are held together in an ever-growing magazine as if they were all contributing to one giant, grindstone-like tooth. Wear between opposing (upper and lower) magazines maintains a grinding surface throughout the life of the dinosaur. Rather than having permanent, hard-wearing grinders (as we do), this could be described as a disposable model that relies on constant replacement of individually simpler teeth.
Opposing edges of each cutting blade of teeth have characteristics that ensure efficiency in their cutting action. The inner surfaces of the lower teeth are coated in a thick layer of extremely hard enamel, while the remainder of the tooth is made of softer, bone-like dentine. In contrast, the upper teeth have the reverse arrangement: the outer edge being coated in thick enamel and the remainder of the tooth is composed of dentine. When the jaws are closed, these opposing blades slide past each other: the hard, enamelled leading edge of the lower jaw magazine meets the enamelled cutting edge of the upper teeth in a cutting/ shearing action rather like the blades of a pair of scissors (Figure 27). Once the enamelled edges have passed one another, the enamel edges (unlike scissor blades) then cut against the less resistant dentine parts of opposing magazines in a tearing and grinding action, which is ideal for crushing up tough plant fibres.
The geometry of the grinding surfaces of the upper and lower ‘magazines’ is particularly interesting. The worn surfaces are oblique, the lower surfaces face outward and upward, while the upper teeth have worn surfaces that face inward and downward. This pattern has interesting consequences. In conventional reptiles, the closure of the lower jaw is brought about by a simple hinge effect, with the jaws on either side of the mouth closing simultaneously in what is called an isognathic bite. If this type of bite is proposed for Iguanodon, then it is immediately obvious that the two sets of teeth on either side of the mouth would simply become permanently wedged together: the lower jaws jamming inside the upper ones. This means it is impossible to imagine how the angled wear surfaces could ever have developed in the first place.
For the angled wear surfaces to have developed, there would have had to be some ability of the jaws to move sideways as they closed. This type of movement is achieved in living herbivorous mammals through the development of an anisognathic jaw closure mechanism. This relies on the fact that the lower jaws are naturally narrower than the upper jaws. Special muscles, arranged in a sling on either side of each jaw bone, are capable to controlling the position of the jaw very precisely so that the teeth on one side meet one another and then the lower set is forcibly slid inwards so that the teeth grind against one another. We humans employ this type ofjaw mechanism, especially when eating tough foods, but it is far more exaggerated in some classically herbivorous mammals such as cows, sheep, and goats, where the swing of the jaw is very obvious.
The whole mammalian type of jaw mechanism is dependent upon very complex jaw muscles, a complex nervous control system, and a specially constructed set of skull bones to withstand the stresses associated with this chewing method. By contrast, more conventional reptiles. of which Iguanodon was one, do not have an anisognathic jaw arrangement, lack the complex muscular arrangements that allow the lower jaw to be very precisely positioned (whether they had the nervous system to control such movements is largely irrelevant), and their skulls are not specially reinforced to withstand the lateral forces acting on the skull bones.
Iguanodon appears to present us with a conundrum: it does not fit any of the expected models. Is the anatomy wrong, or was this dinosaur doing something unexpected?
27. Iguanodon teeth and jaws
The lower jaws of Iguanodon are strong, and quite complex, bones. At the front end each lower jaw is clamped to its neighbour by the predentary bone. The teeth are arranged essentially parallel to the length of the jaw, and at the rear there is a tall prong (coronoid process) of bone which acts as an attachment area for powerful jaw-closing muscles, and as a lever to improve the force of closure that can be exerted on the teeth. Behind the coronoid process are a group of tightly clustered bones that support the hinge-like jaw joint. The upper jaws, during biting, would have been subjected not only to vertical forces, created by the upward closure of the lower jaws and teeth against the uppers, but also to sideways forces generated by the lower teeth wedging themselves between the upper teeth as the bite force increased.
Of all forces acting on the skull of Iguanodon, the ones that it is least well-equipped to deal with are sideways forces acting on its teeth. The long snout (the area in front of the eye sockets) has a deep inverted ‘U’ shape in cross-section. To resist sideways forces acting on the teeth, the skull would need to be braced by bony ‘joists’ connecting the upper jaws; this is the arrangement found in living mammals. Without such bracing, the skull of Iguanodon is very vulnerable to splitting along its midline simply because the depth of the cheek bones creates great leverage against the roof of the snout from forces acting on the teeth. Midline breakage of the skull was avoided by the provision of hinges that are arranged diagonally down either side of the skull; these allow the sides of the skull to flex outward simultaneously as the lower teeth force their way between the upper ones. Other features deeper within the skull helped to provide control over the amount of movement that was possible along this hinge (so that the upper jaws did not simply flop around loosely).
This remarkable system I named pleurokinesis (‘side movement’). On the one hand, the system can be seen as a means of avoiding catastrophic failure of the skull during normal biting. However, the pleurokinetic mechanism allows a grinding motion between opposing sets of teeth. This mimics the grinding motion achieved by herbivorous mammals in a totally different way.
This new chewing system could be linked to another important observation concerning dinosaurs such as Iguanodon. Its teeth are recessed (set inwards) from the side of the face. This creates a depression that might have been covered over by a fleshy cheek -another most un-reptilian feature. Given that the upper teeth slid past the lowers to cut up their food, it seems logical to expect that every time they bit through food in the mouth, at least half of it would be lost from the sides of the mouth … unless, of course,
it was caught and recycled in the mouth by some sort of fleshy cheek. So these dinosaurs appeared to be not only capable of chewing their food in a surprisingly sophisticated way, but they also had mammal-like cheeks, and of course to aid the positioning of the food between the teeth before chews, they would have needed a big, muscular tongue (and strong ceratobranchials – the tongue-muscle bones).
Once this new chewing system had been identified, I was able to recognize that the pleurokinesis was not a ‘one-off invention associated with Iguanodon. It was actually widespread among the general group of dinosaurs known as ornithopods, to which Iguanodon belonged. Tracing the general evolutionary history of ornithopods across the Mesozoic Era, it became clear that these types of dinosaur became increasingly diverse and abundant in time. The ornithopods reached their greatest expression in the ecosystems of the latest Cretaceous period, at which time they are often reported as the most numerous of all land-animal fossils recovered. In some parts of the world, ornithopod dinosaurs, represented at this time by duck-billed or hadrosaurian dinosaurs, were exceedingly abundant and diverse: some discoveries in North America hint at herds of hadrosaurs numbering many tens of thousands of individuals. Hadrosaurs had the most sophisticated dental grinders (each of which had as many as 1,000 teeth in them at any one time) and a well-developed pleurokinetic system.
It seems plausible that these dinosaurs became abundant and diverse in large measure because they were very efficient at eating plant food, using the pleurokinetic system. Their evolutionary success was probably a result of their inheritance of the novel chewing mechanism first identified in Iguanodon.