Neuromuscular disorders
Friedreich’s ataxia is an autosomic recessive hereditary disorder with spinocerebellar degeneration and frequently associated with hypertrophic cardiomyopathy (Gottdiener et al., 1982). A mutation of the frataxin gene (FXN) alters the energy production through mitochondrial iron dysmetabolism resulting in mitochondrial damage producing muscle fiber fibrosis (Michael et al., 2006). The cardiomyopathy may precede the neurological manifestations (Alday & Moreyra, 1984).
Association with congenital heart disease
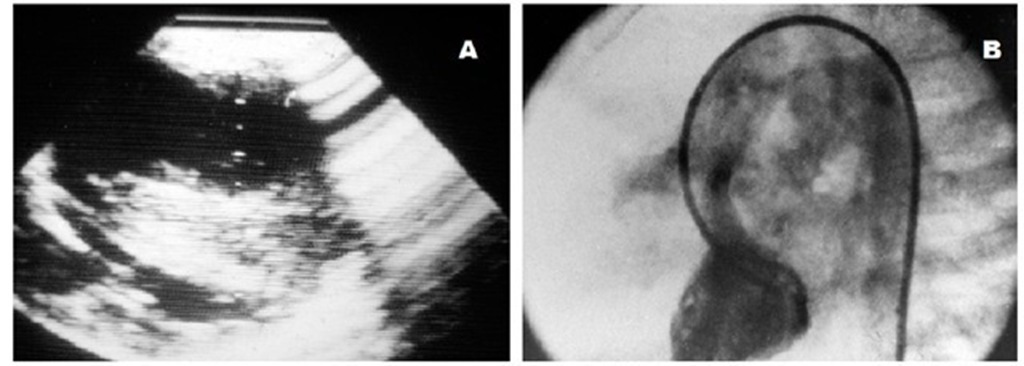
Not infrequently, hypertrophic cardiomyopathy and congenital heart disease are associated in children (Somerville & Becu, 1978). In most circumstances the defects are not severe. Ventricular and atrial septal defects and pulmonary valve stenosis have been reported, the last two mainly in patients with Noonan’s and LEOPARD syndromes (Bruno et al., 2002; Tikanoja et al., 1999). However, associations with severe conditions like tetralogy of Fallot and atrioventricular septal defect have also been found (Alday et al., 1985; Eidem et al., 2000). (Fig. 22)
Association with left ventricular noncompaction
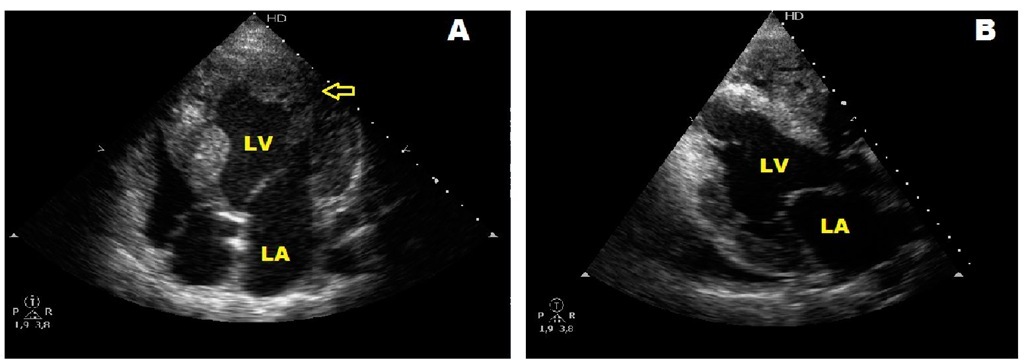
Left ventricular noncompaction belongs to the category of unclassified cardiomyopathies . It is characterized by the presence of prominent myocardial trabeculations and sinusoid tracts mainly in the left ventricle. (Fig. 23) Affected patients frequently develop a dilated cardiomyopathy with heart failure, arrhythmias, and systemic thromboembolism. Recent molecular studies have shown that mutations of genes producing phenocopies like Barth syndrome, or even dilated and hypertrophic cardiomyopathies, are present in patients with left ventricular noncompaction. We recently reported a family with overlapping phenotypes for left ventricular noncompaction, hypertrophic cardiomyopathy, and Wolff- Parkinson-White syndrome. We could not obtain genetic molecular studies but on the basis of features shared with affected patients suspected a mutation of either PRKG2 and LAMP-2 which cause hypertrophic cardiomyopathy with Wolff-Parkinson-White syndrome or Danon’s disease respectively (Alday et al., 2010).
Fig. 22. Two dimensional echocardiogram (A) and left ventricular cineangiography (B) in a patient with tetralogy of Fallot six months after a right modified Blalock-Taussig shunt showing severe ventricular septal hypertrophy, a hypoplastic left ventricle and a dilated aorta overriding a ventricular septal defect.
Fig. 23. A and B. Echocardiographic four-chamber view of two sisters followed since infancy showing hypertrophic cardiomyopathy and associated left ventricular noncompaction. The arrow in A points deep sinusoid tracts. LV: left ventricle, LA: left atrium.
Treatment
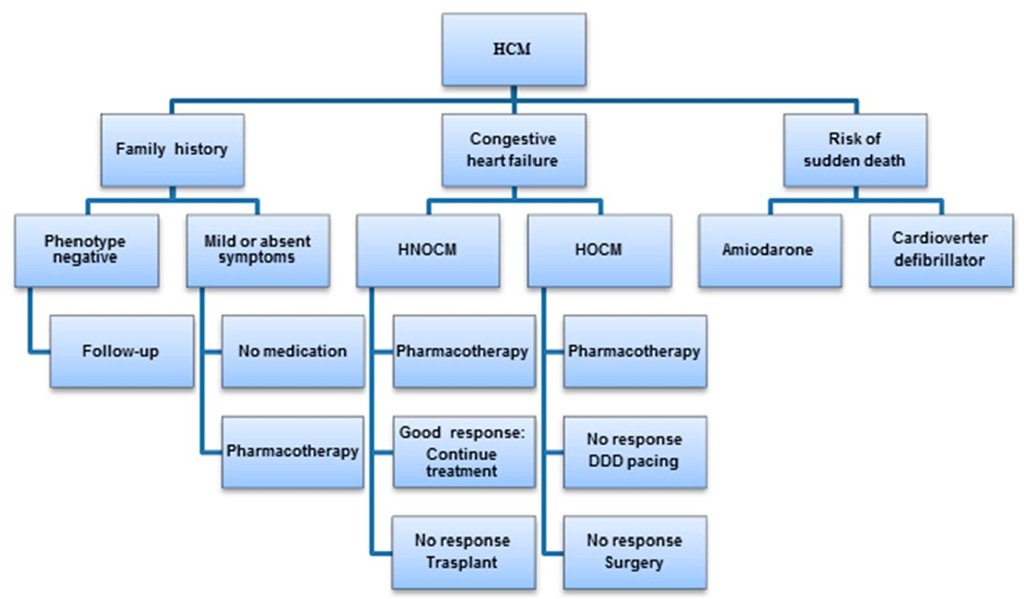
The treatment of hypertrophic cardiomyopathy aims to improve the quality of life alleviating symptoms and to stratify the risk for sudden death to prevent it from happening. Several algorrhythms have been proposed, a slightly modified one is shown in Fig. 24 (Berger et al., 2009). These authors emphasize the existing difficulties to implement prospective controlled randomized trials to define the benefits of different treatment options for this population, therefore most current therapies are empirical or the result of consensus. In older children intensive physical exercise is contraindicated. The disease presenting early has a severe prognosis this being the reason for indicating medical treatment even in asymptomatic children (Bruno et al., 2002). Patients who are symptomatic should receive pharmacological treatment with adrenergic beta blockers which do not decrease the basal outflow gradient but are able to prevent its accentuation in situations of exacerbated inotropism of the heart.
Fig. 24. Algorithm for the treatment of hypertrophic cardiomyopathy
Beta blockers also have anti-ischemic properties which increase ventricular filling by decreasing the heart rate. In these patients, calcium channel blockers like verapamil can be an alternative to beta blockers. It is not recommended to use them together. In patients with severe gradients and pulmonary hypertension are not advised because of the danger of precipitating acute pulmonary edema. If there is no improvement, disopyramide, which is an antiarrhythmic drug with negative inotropic effects, is able to decrease basal gradients. The combination of disopyramide and beta blockers has been used succesfully in adults but there is no reported experience in children (Sherrid et al., (2005). For very symptomatic smaller children, in spite of full medical treatment, permanent DDD pacing with short atrioventricular interval to favor the consistent capture of the right ventricle has been used to lower the left ventricular outflow tract obstruction by changing the pattern of left ventricular activation (Alday et al., 1998 & Fananapazir et al., 1992). DDD pacing is a reasonable alternative to surgery in symptomatic children despite pharmacological treatment if they are still too young for surgery, taking into account that the approach to an extended septal myectomy is just the aortic valve with a small annulus at this age. A very recent study has also shown progressive relief of symptoms and gradient reduction at long term follow-up (Galve et al., 2010). However, as with other types of treatment, DDD pacing does not protect against the possibility of sudden arrhythmic death (Bruno et al., 2002). The gold standard for the treatment of hypertrophic obstructive cardiomyopathy is still the surgical septal myomectomy (B.J. Maron et al., 2003a; Stone et al., 1993). In experienced centers the mortality is very low and the abolition of the gradient is instantaneous and persistent. The remaining obstruction is usually negligible. These excellent hemodynamic results are associated with improvement of symptoms. The results have been followed for many years and the need for repeat procedures is rare. It should be remembered that the child has to be old enough to permit the transaortic approach to the septum (Berger et al., 2009). For this reason, reoperation might be necessary in children 14-year-old or younger (Minakata et al., 2005). With regard to catheter septal ablation with alcohol or radiofrequency the 2003 Expert Consensus Document on Hypertrophic Cardiomyopathy, addressing alcohol septal ablation, states that until the long-term effects of the myocardial scar are known, the procedure is not advised in children (Jensen et al., 2011; B.J. Maron et al., 2003a; Sigwart 1995) In patients with symptomatic hypertrophic nonobstructive cardiomyopathy, calcium antagonists like verapamil could be used to improve the diastolic performance of the left ventricle. Beta blockers are also indicated in this form of the disease. Recently the use of perhexiline which is a metabolic modulator has been introduced for the treatment of patients with this phenotype with improvement of the diastolic performance of the left ventricle and of symptoms (Abozguia et al.; 2010). This was a preliminary report that has still to be supported by further investigations. Infants with heart failure and older children with evolution to a dilated cardiomyopathy should be treated with drugs usually employed for treatment of systolic heart failure. These cases may eventually need heart transplantation (Shirani et al., 1993).
Prevention
Risk stratification of sudden death in infants and children differs from what is done in adults. In children, cardiac death occurs infrequently and non sudden cardiac death is as common as sudden arrhythmic death. The main risk factors for sudden death in children with hypertrophic cardiomyopathy are, according to Maron et al., previous cardiac arrest, syncope, or sustained ventricular tachycardia, family history of sudden death, frequent repetitive non sustained ventricular tachycardia, abnormal blood pressure response to exercise, end-stage hypertrophic cardiomyopathy, and massive left ventricular hypertrophy (B.J. Maron et al., 2003b). Other criteria for death prognostication proposed more recently, specifically in children, take into account the electrocardiographic voltage and echocardiographic parameters like the septal thickness and the left ventricular wall/left ventricular diastolic dimension ratio (Ostman-Smith et al., 2005). For non-sudden cardiac death, massive left ventricular hypertrophy and abnormal blood pressure response to exercise are considered significant risk factors for mortality (Decker et al., 2009). Sudden cardiac death due to hypertrophic cardiomyopathy occurs mostly in adolescence and early adulthood and very infrequently before 10 years of age. These cases are due to ventricular tachycardia or ventricular fibrillation. In fact, hypertrophic cardiomyopathy is the most common cause of sudden death in the young including athletes (J. Seidman & C. Seidman, 2001). This is the reason why the diagnosis of hypertrophic cardiomyopathy at this age is a strong indication to discontinue the practice of competitive sports. At this time it seems that to establish a prognosis by the knowledge of the specific disease causing mutation is not reasonable for the individual patient. Implantable cardioverter defibrillators are effective to prevent arrhythmic sudden death but in infants and children are indicated mainly in secondary prevention (B.J. Maron, et al., 2000b; Epstein, A.; et al., 2008). The cardioverter defibrillator implantation is plagued with complications in children, this being the reason for the reluctance of its use in primary prevention (Berul et al., 2008). In a nonrandomized controlled trial amiodarone was at one time reported to improve survival in hypertrophic cardiomyopathy associated with ventricular tachycardia (McKenna et al., 1985). However, the frequent toxic effects of amiodarone might counteract its benefits (Berger et al., 2009). It then could be concluded that the properly functioning cardioverter defibrillator is nowadays the only effective treatment for the prevention of sudden arrhythmic death (B.J. Maron, et al., 2000b).
Screening strategies
It is well known that hypertrophic cardiomyopathy is a genetic disease of the sarcomeric proteins with great heterogeneity of genetic basis and fenotypic expression which does not only involve these structures but also include abnormalities of the connective tissue, mitral valve, and intramural coronary arteries. The genetic defect may be influenced by modifiers genes and unknown enviromental factors. The strategy for clinical screening for hypertrophic cardiomyopathy with 12 lead electrocardiogram and echocardiogram in non affected family members including < 12 year-old children, is optional, unless there is history of early death due to hypertrophic cardiomyopathy or other serious complications in the family, or is an athlete in training, or evidence of incipient left ventricular hypertrophy, or onset of suspicious symptoms. In family members 12 to 18 years of age, clinical follow-up should be performed every 12 to 18 months and from then on every 5 years. The period of screening should be extended to adulthood since we now know that certain mutant genes can provoke a disease of rather late onset (B.J. Maron et al., 2004).
Conclusion
Great strides have been made since the rediscovery of hypertrophic cardiomyopathy in the late 50′s last century. Important advances in the understanding of the genetics and physiopathology of the disease have occurred as well as development of superb imaging technologies. Treatment is tailored according to the phenotype and stage of the disease. The very important differences between adult and childhood hypertrophic cardiomyopathy have been underlined in this topic hoping that will help physicians in decision making when dealing with these patients.