Blood-brain barrier (BBB) The blood-brain barrier is a collection of cells that press together to block many substances from entering the brain while allowing others to pass. It is a specialized arrangement of brain capillaries that restricts the passage of most substances into the brain, thereby preventing dramatic fluctuations in the brain’s environment. It maintains the chemical environment for neuron functions and protects the brain from the entry of foreign and harmful substances. It allows substances in the brain such as glucose, certain ions, and oxygen and others to enter, while unwanted ones are carried out by the endothelial cells. It is a defensive system to protect the central nervous system.
What is little understood is how the blood-brain barrier is regulated, or why certain diseases are able to manipulate and pass through the barrier.
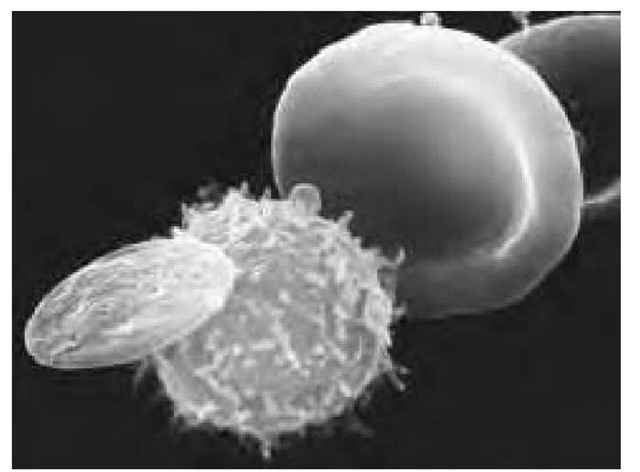
Scanning electron micrograph (SEM) showing three types of cells found in human blood. At right is a red blood cell (erythrocyte), a biconcave disc that transports oxygen around the body. A white blood cell (center) is roughly spherical with microvilli projecting from its surface. Different types of white cells are active in the body’s immune response to infection. The waferlike cell at left is a blood platelet, which functions to control clotting and thus prevents bleeding from damaged vessels. Each cubic millimeter of blood contains approximately 5 million red cells, 7,000 white cells, and 250,000 platelets. Magnification: x3,850 at 35-mm size, x27,000 at 8 x 10-in. size.
There is evidence that multiple sclerosis attacks occur during breakdowns of the blood-brain barrier. A study in rats showed that flavinoids, such as those found in blueberries and grape seeds among others, can inhibit blood-brain barrier breakdown under conditions that normally lead to such breakdown.
Researchers at the University of Maryland School of Medicine in Baltimore have identified a receptor in the human brain that regulates the interface between the bloodstream and the blood-brain barrier and could lead to a new understanding of this nearly impenetrable barrier and to treatment of diseases that affect the brain. They found that two proteins, zonulin and zot, unlock the cell barrier in the intestine, attach themselves to receptors in the intestine to open the junctions between the cells, and allow substances to be absorbed. The new research indicates that zonulin and zot also react with similar receptors in the brain, suggesting that it may become feasible to develop a new generation of drugs able to cross the blood-brain barrier.
Blood Identification through the Ages
by John C. Brenner and Demetra Xythalis
Blood is a fluid that circulates throughout the body, transporting oxygen, nutrients and waste materials. Blood is composed of various formed elements such as red blood cells (erythrocytes), white blood cells (leukocytes), platelets (thrombocytes), and a liquid fraction called plasma, each containing a vast array of biochemical constituents. Red blood cells comprise the majority of the formed elements in the blood. Hemoglobin is a chemical that is found in red blood cells, consisting of an iron-containing pigment, heme, and a protein component, globin. The components of blood are controlled genetically and have the potential of being a highly distinctive feature for personal identification.
The field of forensic science is the study and practice of the application of natural sciences for the purpose of the law. One of the disciplines in forensic science is forensic serology, which involves the identification and characterization of blood and body fluids, either in a liquid or dried state, in association with a criminal or civil investigation. Blood and dried bloodstains are two of the most important and most frequently encountered types of evidence in criminal investigation of crimes such as homicides, assaults, and rapes.
Since the 1900s, forensic serologists have attempted to identify blood and/or bloodstains found at crime scenes. When serology was in its early stages, stains at crime scenes were identified just as blood. Now that forensic serologists can individualize human blood by identifying all of its known factors, the result could be evidence of the strongest kind for linking a suspect to the crime scene or finding a lost victim.
When examining dried bloodstains, the forensic serologists are trying to determine the following: (1) Is the stain blood? (2) If the stain is blood, is it human or animal? (3) If the stain is human blood, can it be associated with a particular individual? The forensic serologist, in an attempt to do blood identification, uses two categories of tests: the presumptive test, which is nonspecific for blood, and the confirmatory, which is specific for blood species.
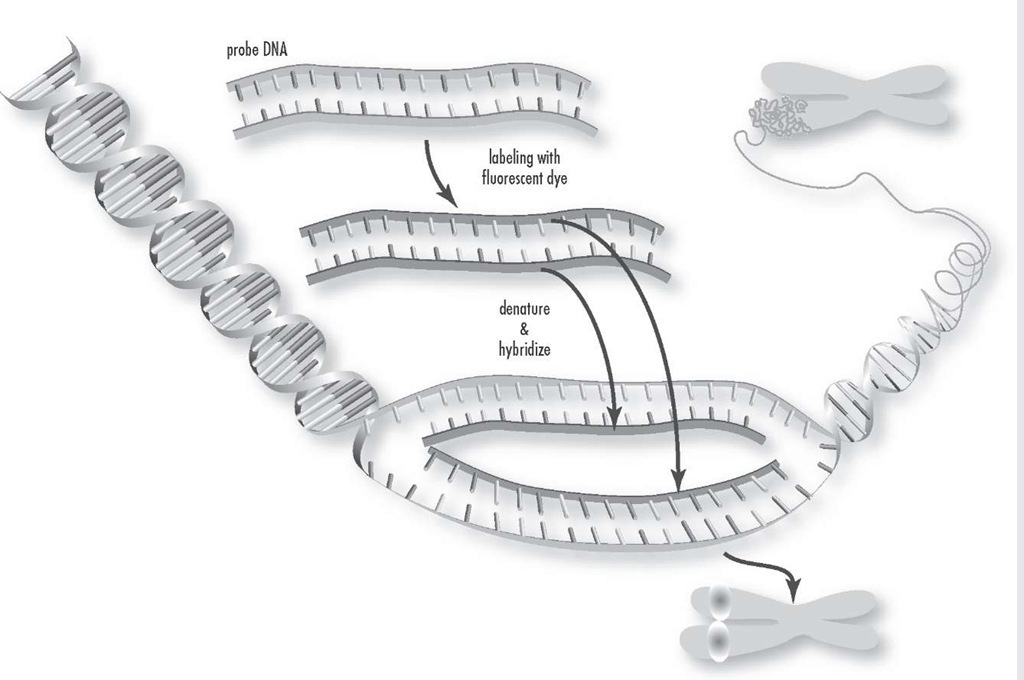
Fluorescence in situ hybridization is a process that vividly paints chromosomes or portions of chromosomes with fluorescent molecules. This technique is useful for identifying chromosomal abnormalities and gene mapping.
The first step in determining whether a crime scene stain is blood involves the use of a chemical screening test or presumptive test. Some presumptive tests used by forensic serologists include benzidine (introduced in 1904), phe-nolphthalein (1901), leucomalachite green (1904), and luminol (1928).
The identification of benzidine as a carcinogen led to its discontinuance as a screening test for blood. Another chemical used for screening stains for blood is phenolph-thalein. Both tests consist of a two-step procedure. The first step is to moisten a white filter paper with distilled water. Apply the filter paper to the suspected bloodstain. A portion of the stain will transfer onto the moistened paper. Add the leucomalachite green reagent to the paper. The second step is to add hydrogen peroxide to the filter paper and look for a color change on the paper. A positive result will yeild a bluish-green color. When phe-nolphthalein is mixed with a dried bloodstain, with the addition of hydrogen peroxide, the hemoglobin in the blood causes the formation of a deep pink color. The leucomalachite green test is a presumptive test for blood that is used by many laboratories today. The heme group in hemoglobin catalyzes the oxidation by peroxide of the malachite green to produce a bluish-green reaction when the suspected stain is blood.
Luminol is a chemical reagent that is very useful in locating small traces of blood at a crime scene. Unlike the above-mentioned chemical screening test, the reaction of luminol with blood results in the production of light rather than color. The one requirement for the use of luminol is that the scene be completely dark. Luminol is a chemical that can be used as a spray at crime scenes and will react with any blood present, causing a luminescence.
The second step in blood identification is the use of confirmatory tests. Confirmatory blood identification tests are specific for the heme component of hemoglobin. A positive confirmatory test result is taken as positive proof of the presence of blood in a questioned stain. Some of the confirmatory tests include: microcrystalline tests, Teichmann and Takayama tests, ring precipitin test, gel diffusion method, and electrophoresis (1907). The Teichmann (1853) and the Takayama (1905) confirmatory tests are based on the observation that heme, in the presence of certain chemicals, will form characteristic crystals that can be seen using a microscope.
The precipitin test is based on antibody molecules interacting with antigens to form a precipitate that can be visualized under the proper light conditions or with a stain. Serologists use this test to determine whether the origin of the bloodstain is human or animal. The ring precipitin test involves layering a dilute saline extract of the bloodstain on top of the antihuman serum in a capillary tube. Because of the density of the antihuman serum, the bloodstain extract will layer on top, and the two solutions will not mix, thus forming a cloudy ring or band at the interface between the two solutions.
The gel diffusion method is based on the fact that antigens and antibodies will diffuse, or move toward each other, on an agar-gel-coated plate, such as the Ouchterlony plate. The extracted bloodstain and the human antiserum are placed in separate holes opposite each other on the gel. A white precipitate line will form where the antigens and antibodies meet if the bloodstain is of human origin.
The electrophoretic method, or crossover elec-trophoresis, is a sensitive method using an electric current that is passed through a gel plate. A line of precipitation formed between the hole containing the bloodstain extract and the hole containing the human antiserum denotes a specific antigen antibody reaction.
The next generation of confirmatory test, which in some forensic laboratories has replaced the electrophoret-ic or crossover electrophoresis is called the One Step ABAcard HemaTrace. This test utilizes a combination of monoclonal and polyclonal antibody reagents to selectively detect human hemoglobin. Adding a portion of the prepared elution from the bloodstain to the sample well and observing the development of indicative colored lines conduct the test. The species specificity of the reaction is based on the recognition by antibodies of antigens displayed on human hemoglobin. A positive result is visualized because gold-conjugated, monoclonal antibody-Hb immune complexes are captured and condensed on the test mambrane by stationary phase polyclonal anti-human hemoglobin antibodies, causing the gold particles to condense. This produces a pink-colored line at the test area. Absence of this colored line indicates a negative result.
There are many different substances in human blood that can be grouped to individualize the blood. Blood contains many inherited factors referred to as genetic markers. Determination of factors in a person’s blood is called blood grouping or blood typing. True individualization of a specimen of blood would mean that a sufficiently large number of genetic markers could be typed so that nobody else in the world would have that particular combination of genetic markers.
In 1900, Dr. Karl Landsteiner announced one of the most significant discoveries of the 20th century—the typing of human blood. Out of Landsteiner’s work came the classification system that is called the ABO system. Blood group systems are the most well known and widely recognized class of genetic markers.
Bloodstains can be typed for ABO using two different procedures: (1) by detecting the antibodies of the serum or (2) by detecting the antigens of the red cells. Detection of the antibodies is the older method. This procedure was first extensively employed by Lattes in Italy in 1913. The procedure has been modified and improved with the development of new antisera. In this test, which detects antibodies in dried bloodstains, two portions of the stains are placed onto microscope slides. Type A red cells are added to one glass slide, and Type B red cells to the other slide. If the bloodstain on the slide contains anti-A antibodies, the A red cells that were added will agglutinate, which looks like crosslinked cells under the microscope. Agglutination of the A cells indicates that anti-A is present, and therefore, that the bloodstain is of blood group B.
The other approach to typing dried bloodstains is the detection of the antigens that are on the surface of the red blood cells. When blood dries, the red cells break apart, but the red blood cell antigens are still present in the dried stains. The two major methods that have been used are absorption-inhibition and absorption-elution. The absorption-inhibition method depends on the ability to estimate the amount of antibody present in an antiserum before and after exposure to a stain extract containing a possible antigen.
The absorption-elution method is based on the theory that blood-group antibodies can bind to their specific red-cell surface antigens in bloodstains. The antigen-antibody complex can then be dissociated and the antibodies recovered. The brea king of the antigen-antibody bond ca n be done by increasing the temperature. Removing specific antibodies from complexes with their antigens in this way is called elution.
Another main class of blood constituents used as genetic markers is the polymorphic enzymes. The enzymes of interest to the forensic serologists are primarily located within the red blood cell and are commonly referred to as isoenzymes. These enzyme forms can be grouped from a bloodstain to further individualize the blood. Red-cell isoen-zymes are frequently typed by a procedure called electrophoresis. This procedure brings about the separation of different proteins based primarily upon differences in net charge, and it is usually done with some kind of starch-gel or on a cellulose acetate support. The most important enzyme systems used in forensic serology are phosphoglu-comutase (PGM), erythrocyte acid phosphatase (EAP), esterase D (ESD), adenylate kinase (AK), adenosine deaminase (ADA), and glyoxalase I (GLO).
The main features of the molecular architecture of deoxyribonucleic acid (DNA) were first formulated by Watson and Crick in 1953, who at the same time pointed out how the proposed structure would account for the three basic attributes of genetic material: gene specificity, gene replication, and gene mutation. It was not until 1985 that forensic scientists discovered that portions of the DNA structure of certain genes are as unique to an individual as their fingerprints. Alec Jeffreys and his colleagues at Leicester University, England, who were responsible for these revelations, named the process for isolating and reading these DNA markers "DNA fingerprinting." The DNA typing of biological fluid and stains finally gives the forensic scientist the ability to link crime scene evidence such as bloodstains to a single individual.
The separation of DNA fragments of different sizes usually can be efficiently accomplished by agarose gel electrophoresis. The agarose gels are thick, which makes them difficult to process in terms of hybridization, washing, and autoradiography. To overcome these problems, a transfer technique was developed that transferred the DNA fragments from the agarose gel onto a nylon membrane. This technique was first described by E. Southern in 1975, and it is called Southern blotting. If a specific recognition base sequence is present, the restriction enzyme recognizing that site will cleave the DNA molecule, resulting in fragments of specific base-pair lengths. Restriction fragment length polymorphism (RFLPs) generates different DNA fragment lengths by the action of specific endonucle-ases. To visualize the separate RFLPs, a nylon sheet is treated with radioactively labeled probes containing a base sequence complementary to the RFLPs being identified, a process called hybridization. Once the radioactive sequences are on the nylon membrane, the membrane is exposed to a piece of X-ray film. The developed X-ray film shows DNA fragments that combined with radioactive probe. The size of the bloodstain (i.e., the amount of blood required) on forensic evidence and the time required to obtain the DNA information from the evidence were two drawbacks to this procedure.
As the push to individualize forensic bloodstain proceeded, the next advancement came in 1983, with molecular biologist Kary Mullis’s development of a process called polymerase chain reaction (PCR). PCR has revolutionized the approach to the recovery of DNA from a variety of sources. Availability of oligonucleotide primers is the key to the amplification process. PCR consists of three steps, beginning with the denaturing of the double-strand DNA, separated by heating to 90-96°C. The second step involves hybridization or annealing, in which one primer is annealed to the flanking end of each DNA target sequence complementary strand. The third step uses a thermally stable Taq polymerase to mediate the extension of the primers. The result is two new helices in place of the first, each one composed of the original strands plus its newly assembled complementary strand.
All eukaryotic genomes contain regions of simple repetitive DNA, called short tandem repeats (STR) or microsatellites, which consist of variable numbers of tandem repeats (VNTRs). The number of repeats at an STR locus can be highly variable among individuals, resulting in different-length polymorphisms that can be detected by relatively simple use of the PCR-based assays. STR loci are useful to forensic science because of their small range of alleles, their high sensitivity, and suitability even if the DNA is degraded. Today the forensic laboratory using the PCR/STR analysis can individualize bloodstains obtained from forensic evidence with a very high probability of identifying a single individual.
In the past, forensic scientists who handled biological forensic evidence were only able to tell the investigating official whether the dried stain at the crime scene was blood. Today the forensic laboratory reports contain information about dried stains at crime scenes that can be related to one individual. This information has been tremendously helpful in the investigation of crimes. With the establishment of a DNA database, physical evidence collected from the crime scene that contains biological stains can be analyzed even if there is no suspect. The DNA profile developed from forensic bloodstain evidence can be compared with various DNA databases to develop a match, which could lead to identification of an individual.
Blood pressure The hydrostatic force that blood exerts against the wall of a blood vessel. This pressure is greatest during the contraction of the ventricles of the heart (systolic pressure), which forces blood into the arterial system. Pressure falls to its lowest level when the heart is filling with blood while at rest (dias-tolic pressure). Blood pressure varies depending on the energy of the heart action, the elasticity of the walls of the arteries, and the volume and viscosity (resistance) of the blood. Blood pressure rises and falls throughout the day.
When the blood flows through the vessels at a greater than normal force, reading consistently above 140/90 mm Hg (millimeters of mercury), it is called hypertension or high blood pressure. High blood pressure strains the heart; harms the arteries; and increases the risk of heart attack, stroke, and kidney problems. About one in every five adults in the United States has high blood pressure. Elevated blood pressure occurs more often in men than in women, and in African Americans it occurs almost twice as often as in Caucasians. Essential hypertension (hypertension with no known cause) is not fully understood, but it accounts for about 90 percent of all hypertension cases in people over 45 years of age.
Low blood pressure is called hypotension and is an abnormal condition in which the blood pressure is lower than 90/60 mm Hg. When the blood pressure is too low, there is inadequate blood flow to the heart, brain, and other vital organs.
An optimal blood pressure is less than 120/80 mm Hg.