Disorders of Neutrophil Function
In patients who have recurrent, severe, or unusual infections but who have a normal number of neutrophils, the presence of a neutrophil function disorder must be considered. Neutrophil function disorders are caused by defects in neutrophil adherence, chemotaxis, degranulation, or oxidative metabolism [see Table 5].
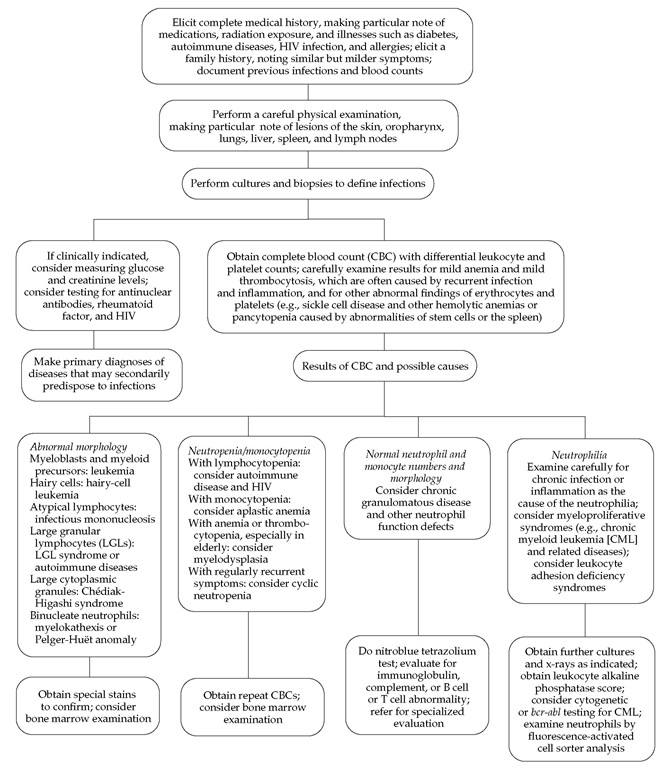
The evaluation of patients with confirmed, recurrent, or un usual infections is first to review the family history and then to examine the patient [see Figure 5 ]. A complete blood count and examination of the granulocytes in a blood smear can show neu-trophilia or neutropenia, specific granule deficiency, or giant granules such as those that occur in Chediak-Higashi syndrome. Evaluation of immunoglobulin levels (IgG, IgM, IgA, and IgE) and complement levels (C3 and CH50) are also potentially helpful, especially if there is a pattern of infection by encapsulated bacteria or unusual organisms. After these considerations, neu-trophil function should be evaluated with the nitroblue tetrazoli-um (NBT) test, superoxide production assays, and chemotactic assays. The NBT test and superoxide assays can determine whether a patient has chronic granulomatous disease (CGD), severe glucose-6-phosphate dehydrogenase (G6PD) deficiency, or a glutathione-pathway disorder14; chemotactic assays can be used to confirm the diagnosis of Chediak-Higashi syndrome and acquired chemotactic defects.12 Leukocyte adhesion deficiency types I and II are diagnosed by flow cytometry.11 If the results of all of these tests are normal, ingestion assays using the patient’s serum and cells and staining for MPO may be helpful. In this manner, all of the known neutrophil function abnormalities can be diagnosed, often with the aid of specialty consultations and a research laboratory.
Monocytes and Macrophage Physiology
|
Table 4 Guidelines for Management and Prevention of Febrile Neutropenia43 |
|
Management |
|
Take careful history and conduct thorough physical examination of the patient |
|
Examine patient carefully for portal for bacterial or fungal infections |
|
Culture blood and other appropriate body fluids |
|
Start antibiotics immediately |
|
Monotherapy (e.g., ceftazidime or imipenem) or duothera-py (e.g., an aminoglycoside, such as gentamicin, with a |-lactam drug that is effective against Pseudomonas, such as piperacillin |
|
Add vancomycin if there is a significant risk of gram-positive sepsis |
|
Adjust antibiotic therapy after 3 days, depending on the results of cultures and the patient’s clinical status Switch low-risk patients to oral therapy |
|
Continue broad-spectrum therapy for severely ill patients* |
|
Consider antifungal treatments |
|
Consider colony-stimulating factors as an adjunct to antibiotics for febrile neutropenia in severely ill, high-risk patients+ |
|
Prevention |
|
Primary prophylaxis with G-CSF reduces incidence of febrile neutropenia by ~50% when the risk of febrile neutropenia is ~40%t |
|
Use G-CSF or GM-CSF as a preventive strategy for patients who have had their treatment reduced or experienced a delay in treatment because of an episode of febrile neutro-penia or a prolonged period of neutropenia Consider reducing the intensity of chemotherapy |
Note: further information can be found at the following Web sites: www.idsociety.org,www.asco.org, and www.guidelines.gov. *Resolution of illness generally follows resolution of neutropenia. +For most patients with febrile neutropenia, CSF therapy has no proven benefit.
tAdministration of G-CSF or GM-CSF is not routinely indicated in previously untreated patients.
G-CSF—granulocyte colony-stimulating factor
GM-CSF—granulocyte-macrophage colony-stimulating factor phages perform tissue maintenance functions, such as clearance of particles—including bacteria—from the blood and removal of old red blood cells. They process antigens by interacting with T cells and B cells and are essential for containment of mycobacte-rial, parasitic, fungal, and viral infections.
Monocyte-macrophage development
Monocytes develop from hematopoietic progenitor cells in the bone marrow. Once the progenitor cells are committed to a monocyte lineage, they develop morphologically into mono-blasts, then promonocytes, and then monocytes. Monocytes are present in the bone marrow and blood. They are the precursors for the tissue mononuclear phagocyte system (including alveolar, peritoneal, and splenic macrophages), Kupffer cells, osteoclasts, dendritic cells, and Langerhans cells. In addition to having phagocytic capabilities, monocytes and macrophages play a central role in the immune response through the generation of numerous cytokines, including growth factors for white blood cells.
With the exception of the alveolar macrophages, which are uniquely dependent on aerobic metabolism for energy production, monocytes and macrophages are facultative anaerobes. Phagocytosis by monocytes and macrophages is associated with an oxidative burst and stimulation of the hexose monophos-phate shunt. Adhesion, chemotaxis, and activation are similar for monocytes and neutrophils, although macrophages are better than neutrophils at phagocytosis and perform chemotaxis less rapidly and efficiently. Macrophages are also capable of oxygen-independent bactericidal activity that may depend on lytic activity. Stimulated macrophages are capable of producing nitric oxide. Macrophages are capable of secreting many cytokines, growth factors, and acute-phase reactants.
Monocytes and macrophages present antigen to T cells in association with major histocompatibility complex (MHC) class II molecules. This association occurs in the lysosomes of a mononuclear cell before the MHC class II molecules are expressed on the cell surface. Monocytes and macrophages are involved in antibody-dependent and antibody-independent cell-mediated cytotoxicity. The cytotoxicity involves oxidative metabolism, the production of nitric oxide and cytokines, and the secretion of cytotoxic mediators.
Macrophages play a key role in metabolizing high-molecular-weight proteins, glycoproteins, and other material and are intimately involved in the destruction of senescent and killed cells. They also are required for angiogenesis and wound healing and are able to induce neovascularization and endothelial cell proliferation. Given these diverse products and functions, macro-phages are involved in many metabolic, infectious, inflammatory, and degenerative diseases.
Increases in blood monocytes (usually less than two times the normal level or less than 1.0 x 109/L) are a common feature of chronic inflammatory diseases and malignancies. Higher counts should raise concern about a hematologic malignancy (e.g., monocytic or myelomonocytic leukemia) [see 12:XVI Acute Leukemia].
Disorders of Monocytes and Macrophages
Histolytic Syndromes
Histiocytic syndromes are a group of malignant and nonma-lignant disorders in which the macrophages and dendritic (Langerhans) cells are the principal cells of abnormality.49 The malignant disorders include acute monocytic leukemia, mono-cytic sarcoma, and histiocytic sarcoma. The nonmalignant disorders include the Langerhans cell histiocytosis (LCH) syndromes and the hemophagocytic syndromes, such as sinus histiocytosis with massive lymphadenopathy, hemophagocytic lymphohisti-ocytosis (HL), and infection-associated hemophagocytic syndrome (IAHS).
Langerhans Cell Histiocytosis Syndromes
The LCH syndromes include solitary eosinophilic granuloma, multifocal eosinophilic granuloma, Hand-Schuller-Christian disease, and Letterer-Siwe disease.49,50 These disorders predominantly affect children 1 to 15 years of age but also occur in young adults. The LCH syndromes represent a continuum of disease that has been divided on the basis of histologic studies, age at diagnosis, extent of disease, and organ involvement. The signs and symptoms of the LCH syndromes depend on the specific organs involved.51 The bones, skin, teeth, gingival tissue, ears, endocrine organs, lungs, liver, spleen, lymph nodes, and bone marrow can all be involved and become dysfunctional as a result of cellular infiltration.52 For example, diabetes insipidus is caused by histo-cyte infiltration of the pituitary gland,53 and Erdheim-Chester disease is a multisystem disease characterized by histiocyte infiltration of many tissues.54 LCH with solitary and multifocal eosinophilic granuloma is found predominantly in older children and young adults; more infiltrative disease is common in younger patients.55 On presentation, patients with solitary lesions may have an inability to bear weight, or they may have tender swelling caused by tissue infiltrates that overlie a sharply marginated bony lesion. Diagnosis is usually made by demonstration of dendritic cells, eosinophils, and giant cells present in a biopsy specimen; electron microscopy and immunostaining may be helpful for further classification.
Table 5 Selected Disorders of Neutrophil Function
|
Disorder |
Inheritance |
Clinical Features |
Diagnosis |
Treatment |
|
|
Leukocyte adhesion deficiency I |
AR Locus: 21q22.3 |
Neutrophilia with recurrent severe infections; failure of pus formation; delayed umbilical cord separation |
Decreased neutrophil adherence and migration; CD11/CD18 deficiency Clinical testing available* |
Bone marrow transplantation; antibiotics |
|
|
Adherence defects |
Leukocyte adhesion deficiency II |
S, possibly AR |
Neutrophilia with recurrent infections |
Neutrophils lack surface sLex and have deficient adherence |
Bone marrow transplantation; antibiotics |
|
Actin polymerization defect |
AR, S |
Recurrent severe infections |
Defective neutrophil migration and ingestion of bacteria |
Bone marrow transplantation; antibiotics |
|
|
Granule |
Chediak-Higashi syndrome |
AR, S Locus: 1q42 |
Recurrent infections; partial albinism; lymphoproliferative syndrome; neutropenia; thrombocytopenia |
Giant cytoplasmic granules; defective neutrophil migration and bacterial killing Genetic testing: research only* |
Antibiotics; vitamin C; bone marrow transplantation |
|
defects |
Specific granule deficiency |
S, possibly AR |
Recurrent infections, especially sinopulmonary and skin infections |
Absence of specific (secondary) granules in neutrophils; abnormal neutrophil migration and respiratory burst |
Antibiotics |
|
Respiratory burst defects |
Chronic granuloma-tous disease |
AR or X-linked CYBAlocus: 16q24 CYBB locus: Xp21.1 NCF1 locus: 7q11.23 NCF2 locus: 1q25 |
Recurrent skin, pulmonary, and liver abscesses |
Severely defective respiratory burst; NBT test; abnormality in one of four subunits of NADPH oxidase Clinical testing available* |
Interferon gamma; antibiotics |
|
Glucose-6-phosphate dehydrogenase deficiency |
X-linked Locus: Xq28 |
Recurrent bacterial infections; hemolytic anemia |
Reduced levels of glucose-6-phosphate dehydrogenase Clinical testing available* |
Antibiotics |
|
|
Myeloperoxidase deficiency |
AR |
Mild, if any, susceptibility to infection |
Reduced levels of myeloperoxidase |
Generally none indicated |
AR—autosomal recessive
NADPH—nicotinamide-adenine dinucleotide phosphate
NBT—nitroblue tetrazolium
S—sporadic cases
sLex—sialyl-Lewisx
Treatment of local LCH is sometimes unnecessary; when it is necessary, surgery or local radiation therapy can be cura-tive.50-55 LCH syndromes respond to chemotherapeutic agents, including vinblastine, methotrexate, 6-mercaptopurine, etopo-side, or 2-chlorodeoxyadenosine (cladribine). There is a long-term risk of secondary or treatment-related malignancies in these patients.
Sinus Histiocytosis with Massive Lymphadenopathy
Sinus histiocytosis with massive lymphadenopathy, or Ro-sai-Dorfman disease, is characterized by chronic, painless, massive lymphadenopathy that usually involves the cervical nodes and less frequently involves the axillary, hilar, peritracheal, or inguinal nodes.56 It occurs in both adults and children. Extra-nodal disease in the respiratory tract, bones, orbits, skin, liver,and kidneys is present in almost 30% of patients. The disease is usually benign, but significant morbidity and even death may result if massive tissue invasion of the liver, kidneys, lungs, and other critical structures occurs. Patients are usually of African descent, and the incidence of this disease is highest in Africa and the West Indies.
The affected lymph nodes show marked sinusoidal dilatation and follicular hyperplasia with proliferation of foamy histiocytes and multinucleated giant cells in the sinuses. The etiology of this disorder is unknown and may be related to abnormal immune regulation. Attempts at treatment should be reserved for special circumstances that are potentially life threatening. Surgery, irradiation, corticosteroids, vinblastine, and cyclophosphamide have all been administered with varying degrees of success.
Hemophagocytic Lymphohistiocytosis
HL is a rapidly fatal disorder, occurring as a familial or acquired condition; it is characterized by fever, pancytopenia, hepatic dysfunction, and activated macrophages, which overproduce inflammatory cytokines.57 Family studies suggest that a portion of cases are attributable to mutations in the perforin gene.58,59 The disease is usually diagnosed in young children; however, secondary forms of HL account for numerous cases in adults and occur in association with bacterial, fungal, and parasitic infections and exposure to various drugs. Treatment is difficult. Chemotherapy may be helpful. If the disease is associated with infection, treatment with appropriate antimicrobials may resolve the disorder.
Lysosomal Storage Diseases
Monocytes and macrophages play a role in tissue remodeling and the removal of senescent cellular debris, and lysosomes are the organelles that perform these functions; therefore, enzymatic abnormalities that involve lysosomal constituents result in disorders of storage that are related to macrophage function. These disorders, usually diagnosed in early childhood, include the mu-copolysaccharidoses, the glycoproteinoses, the sphingolipidoses, and the neutral lipid storage diseases [see Table 6]. Enzymatic defects have been described for most of these disorders, and diagnosis depends on demonstrating the enzymatic abnormality in macrophages or histiocytes. Most of these defects result from point mutations or genetic rearrangements at a single locus of the gene that codes for a single lysosomal hydrolase.
Figure 5 Steps in the evaluation of a patient with recurrent infections for a phagocytic cell disorder.
Table 6 Lysosomal Storage Diseases
|
Disease (Common Name) |
Inheritance |
Enzymatic Defect |
Organs and Tissues Involved |
Stored Material |
|
Mucopolysaccharidoses (MPS) |
||||
|
MPS IH (Hurler syndrome) |
AR; locus: 4p16.3 |
a-L-Iduronidase |
Liver, spleen, brain, heart, cornea, bone (mild and severe variants) |
Dermatan sulfate, heparan sulfate |
|
MPS II (Hunter syndrome) |
Locus: Xq28 |
Iduronate-2-sulfatase |
Liver, spleen, brain, heart, bone |
Dermatan sulfate, heparan sulfate |
|
MPS III |
||||
|
(Sanfilippo Asyndrome) |
Locus: 17q25.3 |
Heparan N-sulfatase |
Brain, liver, spleen, heart, bone |
Heparan sulfate |
|
(Sanfilippo B syndrome) |
Locus: 17q21 |
a-N-Acetylglucosaminidase |
Brain, liver, spleen, heart, bone |
Heparan sulfate |
|
(Sanfilippo C syndrome) |
Chromosome 14 |
Acetyl-coenzymeA:a- |
||
|
glucosaminide |
||||
|
N-Acetyltransferase |
||||
|
MPS IV |
||||
|
(Sanfilippo D syndrome) |
Locus: 12q14 |
N-Acetylglucosamine-6-sulfatase |
||
|
(Morquio Asyndrome) |
Locus: 16q24.3 |
N-Acetylgalactosamine-6-sulfatase |
Bone, cornea |
Keratan sulfate, chondroitin 6-sulfate |
|
(Morquio B syndrome) |
Locus: 3p2 |
P-Galactosidase |
Bone, cornea |
Keratan sulfate |
|
MPS VI (Maroteaux-Lamy syndrome) |
Locus: 5q11-q13 |
N-Acetylgalactosamine-4-sulfatase |
Bone, cornea, liver, spleen, heart (moderate and severe variants) |
Dermatan sulfate |
|
MPS VII (Sly syndrome) |
Locus: 7q21 |
P-Glucuronidase |
Brain, liver, spleen, bone, coro- |
Dermatan sulfate, heparan sulfate, |
|
nary arteries |
chondroitin 4-sulfate, chondroitin 6-sulfate |
|||
|
Glycoproteinoses |
||||
|
Mannosidosis |
AR; locus: 19cen-q12 |
Lysosomal a-mannosidase |
Brain, liver, spleen, bone (several variants) |
Mannose-rich oligosaccharides |
|
Fucosidosis |
Locus: 1p34 |
Glycoprotein a-fucosidase |
Brain, liver, spleen, heart, skin |
Fucose-containing oligosaccharides |
|
(several variants) |
||||
|
Aspartylglucosaminuria |
Locus: 4q32-q33 |
Aspartylglucosaminidase |
Brain, liver, spleen, bone, heart |
Aspartylglucosamine-containing peptides |
|
Sialidosis |
Locus: 6p21.3 |
Glycoprotein neuraminidase (sialidase) |
Brain, liver, spleen, bone, retina (several variants) |
Sialylated glycopeptides |
|
Galactosialidosis |
Locus: 20q13 |
Protector protein deficiency, combined neuraminidase (sialidase) and p-galacto-sidase deficiency |
Brain, liver, spleen, bone (several variants) |
GM1 ganglioside, sialylated glycopeptides |
|
Mucolipidosis II (I-cell disease) |
Locus: 4q21-q23 |
N-Acetylglucosamine-1-phosphotransferase |
Brain, bone, connective tissue |
Glycoproteins, glycolipids |
|
Mucolipidosis III (Pseudo-Hurler polydystrophy) |
? |
N-Acetylglucosamine-1-phosphotransferase |
Brain, bone, connective tissue |
Glycoproteins, glycolipids |
|
Mucolipidosis IV (sialolipidosis) |
Locus: 19p13.3-p13.2 |
Mucolipin 1 |
? |
? |
|
Sphingolipidoses |
||||
|
(Gaucher disease type I [nonneuronopathic]) |
AR; locus: 1q21 |
Acid p-glucosidase, glucocerebrosidase |
Liver, spleen, bone, bone marrow (highly variable phenotype) |
Glucosylceramide |
|
(Gaucher disease type 2 [acute neuronopathic]) |
Acid p-glucosidase, glucocerebrosidase |
Brain, brain stem, liver, spleen, bone marrow, lungs |
Glucosylceramide, glucosylsphingosine |
|
|
(Gaucher disease type 3 [sub-acute neurono-pathic]) |
Acid p-glucosidase, glucocerebrosidase |
Brain, liver, spleen, bone marrow, lungs (variable phenotype) |
Glucosylceramide, glucosylsphingosine |
The two types of therapy for lysosomal storage diseases that are currently available are cellular transplantation and enzyme therapy.60 Gaucher disease was formerly treated with bone marrow transplantation, but it is currently treated with alglucerase, an a-mannosyl-terminated glucocerebrosidase. Bone marrow transplantation for the other lysosomal storage diseases is inves-tigational and has yielded mixed results.
Eosinophil Physiology
Eosinophils can enhance or suppress acute inflammatory reactions and mediate responses to helminthic infection, allergy, and certain tumors.61 Like neutrophils, eosinophils are capable of phagocytosis, but eosinophils are primarily secretory cells. Most of the functions they perform require the release of granule contents or reactive oxygen species. The eosinophils respond to unique chemotactic agents and growth factors that permit their accumulation at sites of inflammation.