Leukocytes, or white blood cells, protect the body against infections and participate in many types of immunologic and inflammatory responses. There are two main types of leukocytes: lymphocytes, which are responsible for antibody production and cell-mediated immunity, and phagocytes, which are responsible for the ingestion and killing of microorganisms. Neutrophils, monocytes, macrophages, and eosinophils are all phagocytes [see Figure 1]. Leukocytes interact with one another and modulate immune responses through the release of cytokines (interleukins and growth factors), enzymes, and vasoactive substances. This topic covers the diagnosis of disorders of neutrophils, mono-cytes, and eosinophils and the treatment of neutropenia; the functions and disorders of lymphocytes are discussed elsewhere [see Section 6 Immunology and Allergy].
The White Blood Cell Count
The total white blood cell (WBC) count and differential count are often the first studies performed in evaluating a patient with a suspected infection or with susceptibility to infections. Most laboratories measure the WBC count using automated cell-counting techniques.1 The normal WBC count ranges from 4,300 to 10,000/mm3, with a median of 7,000/mm3 [see Table 1]. A differential count gives the percentage for each type of leukocyte. The absolute count is determined by multiplying the total WBC count by this percentage (e.g., WBC x percent neutrophils = absolute neutrophil count). Because the blood level of each type of leukocyte is separately regulated, it is always better to use the absolute count rather than the percentage in assessing abnormalities.
Indications of the Presence of a Phagocytic Cell Disorder
Because the phagocytes, particularly neutrophils, represent the first line of defense against invading microorganisms, disorders in the number or function of these cells often result in an increased susceptibility to infection. A quantitative or qualitative disorder of phagocytic cells should be suspected when a patient has an increased number of bacterial or fungal infections, increasingly severe infections, or infections with unusual organisms.
Neutrophil Physiology
NEUTROPhIL PRODUCTiON
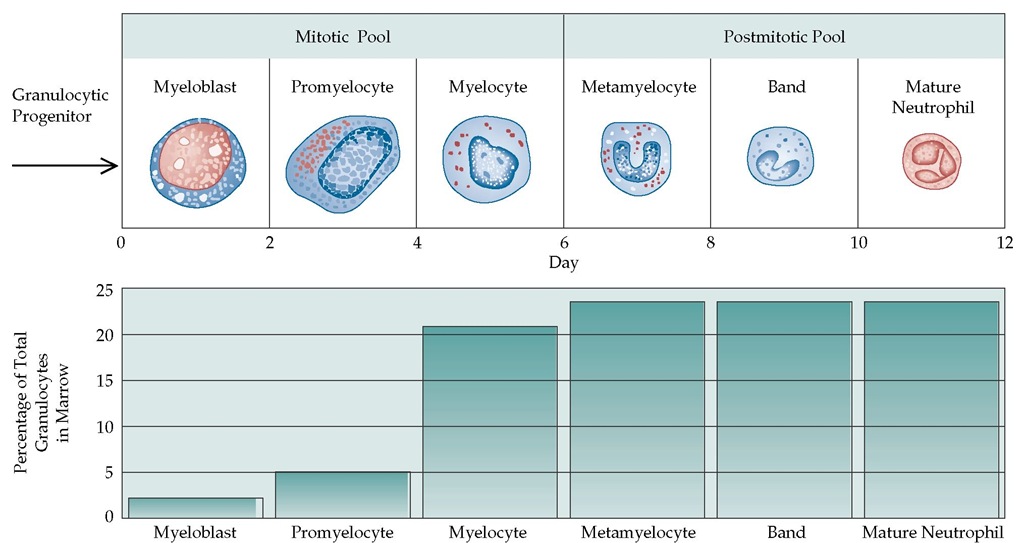
Neutrophils are derived from the common stem cell, which also gives rise to erythrocytes, platelets, and other leukocytes. The proliferation and differentiation of the neutrophil precursors are governed by a family of regulatory cytokines. Granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) are two important cytokines affecting neutrophil production and function. G-CSF selectively stimulates progenitor cells to differentiate into neutrophils and rapidly increases blood neutrophils in hematologically normal individuals [see Figure 2].2,3 GM-CSF stimulates progenitor cells to differentiate into neutrophils, eosinophils, monocytes, macro-phages, and dendritic cells.4 The life cycle of the neutrophil consists of bone marrow, blood, and tissue phases. Neutrophil production in the bone marrow takes approximately 10 to 14 days, and the bone marrow produces approximately 1 x 109 neu-trophils/kg/day.5 Most of the body’s neutrophils are found in the bone marrow. The mitotic compartment, which contains about 20% of the total neutrophil pool, consists of myeloblasts (the earliest morphologically recognizable precursors), promye-locytes, and myelocytes. The postmitotic pool or maturation com-partment—the metamyelocytes, bands, and mature neutrophils—contains about 70% of the body’s neutrophils. The marrow neutrophils and bands are sometimes called the storage compartment or marrow reserve. As neutrophils mature, they develop the capacity to enter the blood through increasing de-formability and through changes in the adhesion proteins on their surface membranes. Entry into the blood involves interactions of the mature cells and the endothelial cells of the marrow sinusoids that are not yet well understood. Agents that stimulate release of neutrophils from the marrow (e.g., G-CSF, GM-CSF, corticosteroids, or endotoxin administration) can result in a doubling of the blood neutrophil count within 3 to 5 hours. The peripheral blood contains fewer than 10% of the body’s neutrophils. In the blood, the neutrophils are divided approximately evenly between the circulating pool and the marginating pool; these pools are in dynamic equilibrium. Cells in the marginating pool can be swept rapidly (within minutes) into the circulation by endogenous or exogenous epinephrine or as a result of exercise or any cause of rapid increase in cardiac output. This response, called demargination, can double the blood neutrophil count very rapidly and is also quickly reversible. The blood half-life of the neutrophils is approximately 6 to 10 hours. Neutrophils leave the blood and enter the tissues by migrating between endothelial cells and penetrating the capillary basement membrane. It is now believed that neutrophils that do not leave the circulatory system die by apoptosis and are removed by mononuclear phagocytes in the spleen, liver, and other tissues.6
NEUTROpHIL STRUcTURE
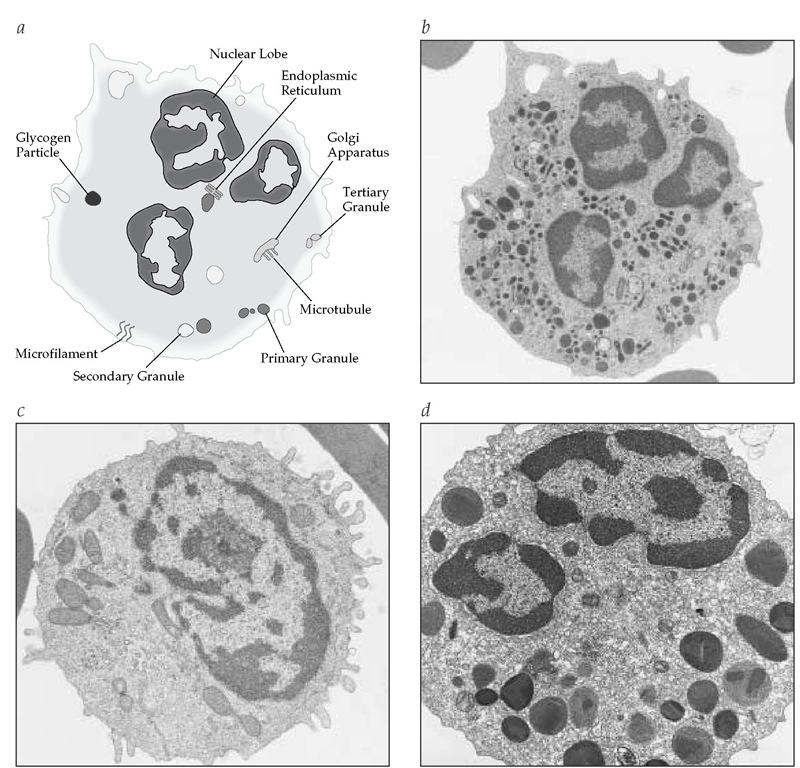
As neutrophil precursors mature, their nuclear chromatin becomes condensed and segmented. Mature cells have no nucleoli, few mitochondria, and very little endoplasmic reticulum. The cytoplasm is filled with granules and glycogen. The primary granules, which appear at the myeloblast and promyelocyte stages, contain myeloperoxidase (MPO), proteases, defensins, and other antibacterial substances.7,8 Secondary granules, produced primarily during the myelocyte stage, predominate in mature cells. They contain collagenase, lactoferrin, lysozyme, vitamin B12-binding protein, and several other proteins. Small tertiary granules are also found in mature neutrophils. Neutrophils also may have cytoplasmic vesicles containing lactases, alkaline phosphatases, and components of nicotinamide-adenine dinu-cleotide phosphate (NADPH) oxidase.
The surface of the neutrophil is replete with deep folds and ruffles. On the neutrophil surface, there are numerous receptors, including receptors for immunoglobulins (e.g., FcyRII [CD32],FcyRIII [CD16]), complement (e.g., CR3 [CD11b18], CR1 [CD35]), chemokines, the colony-stimulating factors G-CSF and GM-CSF, Fas, tumor necrosis factor receptor (TNF-R), and the apoptosis-re-lated receptors.9
Figure 1 Shown are a schematic diagram of a neutrophil (a), a corresponding electron micrograph of a neutrophil (b), and electron micrographs of a monocyte (c) and an eosinophil (d).
The cytoskeleton of the neutrophil is composed of microtubules and microfilaments that are critical for phagocytic shape and movement, including migration through the vascular en-dothelium. The microfilaments, which consist primarily of actin polymers, are dispersed throughout the cytoplasm.10
Neutrophil Function
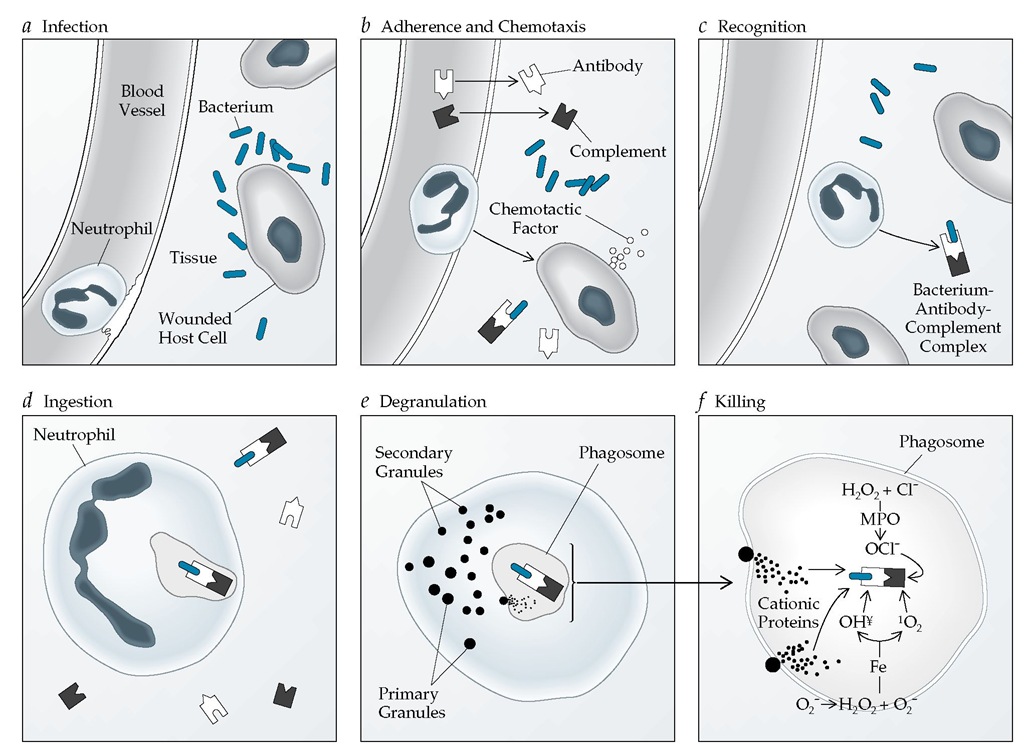
The major function of neutrophils is to respond rapidly to mi-crobial invasion to kill the invaders. This response has several distinct steps—adherence, migration, recognition, phagocytosis (or ingestion), degranulation, oxidative metabolism, and bacterial killing [see Figure 3]. Susceptibility to infection results from abnormalities in any one or a combination of these processes.
Adherence
For neutrophils to move to an inflammatory site, they must first adhere to a capillary wall.11 Loose adherence is facilitated by L-selectins, such as sialyl-Lewisx (sLex), on the neutrophil and E-selectin and P-selectin on capillary endothelial cells [see Figure 4]. Bacterial invasion increases local selectin expression and neu-trophil accumulation. Other neutrophil surface proteins, called |2 integrins, facilitate firmer adhesion to endothelial cells and interact with actin, myosin, and actin-binding proteins to initiate movement of neutrophils to the tissue.11 The three proteins in this family have a common | subunit (CD18) and a different a sub-unit (CD11a, CD11b, or CD11c). There is generally increased expression of these proteins (e.g., CD 11b/C18) on neutrophils in response to inflammation. Concomitantly, there is increased expression of the intracellular adherence molecules (ICAMs) on the endothelial cells, with a net result of increased trafficking of neutrophils to the inflammatory focus.
Table 1 Normal Leukocyte Values in Peripheral Blood
|
Cell Type |
Cells/mm3* |
Percentage of Total Differential Count |
|
|
Median |
Range |
||
|
All leukocytes (white blood cells) |
7,000 |
4,300-10,000 |
100 |
|
Total neutrophils |
4,000 |
1,800-7,200 |
55 |
|
Band neutrophils |
500 |
100-2,000 |
10 |
|
Segmented neutrophils |
3,500 |
1,000-6,000 |
45 |
|
Lymphocytes |
2,500 |
1,500-4,000 |
36 |
|
Monocytes |
450 |
200-900 |
6 |
|
Eosinophils |
150 |
0-700 |
2 |
|
Basophils |
30 |
0-150 |
1 |
*To calculate the number of cells/L, multiply by 106.
Chemotaxis
Chemotaxis, the directed movement of cells, occurs when neutrophils detect a chemoattractant at low concentrations and move up the concentration gradient toward its source, which is usually a site in the extravascular spaces.12 Well-characterized stimulators of neutrophil chemotaxis are the complement proteins C5a, leukotriene B4, interleukin-8 (IL-8), and a family of small peptides, the chemokines. The trafficking of neutrophils from the blood is unidirectional; they do not return from the tissues to the circulation.
Recognition and Phagocytosis
At the site of inflammation, neutrophils utilize their im-munoglobulin and complement receptors to recognize bacteria and other particles coated or opsonized by immunoglobu-lins or complement. Inflammation stimulates neutrophils to express increased numbers of the high-affinity IgG receptor FcyRI (CD64).13As the neutrophil internalizes a particle, a phagocytic vesicle, or phagosome, develops around it. This process stimulates degranulation and activates a burst of ox-idative metabolism.
Degranulation
When the neutrophil is activated, the granule membranes come in contact with the plasma membranes surrounding the phagosome. The membranes fuse, which leads to the release of granule proteins into the phagosome and to the reorganization of the components of the critical NADPH oxidase system.
Oxidative Metabolism and Bacterial Killing
Resting granulocytes are primarily anaerobic cells that rely on anaerobic glycolysis for adenosine triphosphate (ATP) production. Although chemotaxis, ingestion, and degranulation require some energy, they also proceed quite well anaerobically.
Figure 2 The process of neutrophil maturation begins in the bone marrow (top). After about 12 days, approximately 10% of the mature neutrophils are released into the peripheral blood, where they have a half-life of approximately 6 to 10 hours. Eventually, the neutrophils migrate into the tissues by diapedesis. The percentage of neutrophils at each stage of development (bottom) ranges from about 2% at the myoblast stage to almost 25% at the mature neutrophil stage.
Figure 3 The neutrophil response to bacterial invasion involves several stages. A bacterium infects a host cell and injures it (a). Bacterial products, antibodies, and complement cause the release of chemotactic factors, which activate a neutrophil in the adjacent blood vessel. The neutrophil adheres to the vessel wall and undergoes chemotaxis and diapedesis into tissue (b) to follow the chemoattractants to their sites of generation or expression. The neutrophil recognizes (c) and ingests (d) the bacterium-antibody-complement complex, forming a phagosome. The neutrophil then undergoes degranulation, a process in which granule membranes fuse with the plasma membrane (e). Degranulation releases various enzymes and enhances oxidative metabolism, the products of which are bactericidal (f). For example, hydrogen peroxide (H2O2), produced from superoxide (O2 ), can interact with O2- in the presence of iron (Fe) to produce hydroxyl radicals (OH^) and singlet oxygen (1O2), both of which are highly toxic to bacteria. In addition, H2O2 and chloride (Cl-) combine in the presence of the myeloperoxidase (MPO) released in the phagosome to produce hypochlorite (OCl-), which is also bactericidal.
However, bacterial killing generally is associated with a rapid increase (within seconds) in oxygen use. This respiratory burst occurs as a result of the activation of an NADPH oxidase.15 Before activation, the components of the oxidase are located separately in the plasma and granule membranes and in the cytosol. The membranes contain two components: gp91phox and p22phox. The cytosol includes a p47 protein and a p67 protein. When the neu-trophil is activated, the cytosolic proteins first associate and then combine with the membrane components to produce the complete NADPH oxidase. NADPH oxidase can reduce oxygen by one electron to superoxide O2-; in the process, NADPH is converted to NADP+. The NADPH is then regenerated through the hexose monophosphate shunt. Dismutation of the superoxide in the presence of superoxide dismutase produces hydrogen peroxide (H2O2), which can then be converted to hydroxyl radical (OH^). H2O2, O2-, and OH are highly toxic. In addition, within the phagocyte vacuole, hydrogen peroxide and chloride (Cl-) can combine in the presence of myeloperoxidase to produce hypochlorous acid (HOCl), which is bactericidal.15 These products of the respiratory burst can also be released from the activated neutrophil and subsequently damage the surrounding cells and tissues.
Responses to and Production of Cytokines
The growth factors that affect neutrophil production, such as G-CSF and GM-CSF, also influence neutrophil function.16 These cytokines upregulate stimulus-dependent NADPH oxidase activity and can enhance bactericidal and fungicidal activities. Although neutrophils contain very few ribosomes, they can respond to bacterial stimuli by synthesizing and secreting proin-flammatory cytokines such as IL-1, IL-6, and tumor necrosis factor-a (TNF-a); monocytes, however, produce much larger quantities of these substances.