Angiotensin system polymorphisms
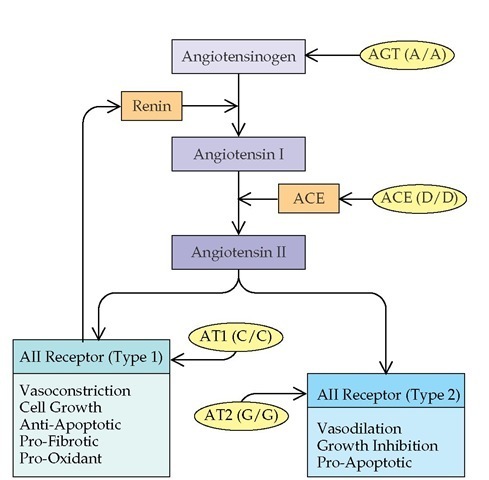
The renin-angiotensin system is a metabolic-hormonal pathway that plays a critical role in blood pressure homeostasis and salt and water balance. In the renin-angiotensin pathway, the prohormone angiotensinogen (AGT) is converted to angioten-sin I by renin.
Table 2 Renin-Angiotensin System and Chemokine Polymorphism and Pathophysiology26 60-72
|
Locus |
Position |
Genotype |
Pathophysiologic Effect |
|
ACE |
Deletion variant |
I/I or I/D |
Favorable function trend, pediatric renal transplants |
|
ACE |
Deletion variant |
D |
Favorable renal function, bone marrow transplant patients |
|
ACE |
Deletion variant |
D/D homozygotes |
Favorable renal function, renal transplants |
|
ACE |
Deletion variant |
D |
Increased cardiac allograft vascular disease |
|
ACE |
Deletion variant |
D/D homozygotes |
Increased risk of renal failure, pediatric kidney transplants |
|
ACE |
Deletion variant |
D/D homozygotes |
Inreased risk of renal failure, high-risk renal transplants |
|
ACE |
Deletion variant |
D |
Increased frequency in systemic lupus erythematosus |
|
ACE |
Function |
Function |
ACE inhibitor suppresses IL-12, IFN-y |
|
ACE |
Deletion variant |
D/D homozygotes |
Increased ACE levels |
|
ACE |
Deletion variant |
D/D homozygotes |
Increased blood pressure with salt administration |
|
ACE |
Deletion |
D |
Increased risk of renal dysfunction, renal transplants |
|
AGT |
Met235Thr |
Met vs Thr |
No function association, pediatric renal transplants |
|
AGT |
G ^ A,-6 |
A/A |
Increased risk of renal dysfunction, renal transplants |
|
AT1 |
A1166C |
A vs C |
No function association, pediatric renal transplants |
|
AT1 |
Function |
Function |
Activation of NF-kB via IL-12 and IFN-y |
|
AT1 |
A1166C |
C |
Increased blood pressure, renal transplant patients |
|
CCR2 |
V64I |
I |
Less acute rejection, renal transplants |
|
CCR2 |
Expression |
Expression |
High expression in renal transplants during rejection |
|
CCR5 |
A59029G |
A/A homozygotes |
Less acute rejection, renal transplants |
|
CCR5 |
Expression |
Expression |
High expression in renal transplants during rejection |
|
CXCR3 |
Expression |
Expression |
High expression in heart biopsy infiltrates |
Note: Some of the gene variants appear to have paradoxical effects, depending on the investigator and the assay system. ACE—angiotensin-converting enzyme IFN—interferon IL—interleukin
Angiotensin-converting enzyme then catalyzes the conversion of angiotension I to angiotensin II [see Figure 4]. Angiotensin II is one of the most potent vasoconstrictive human hormones. In addition, angiotensin II has indirect inflammatory and fibrotic effects, which are distinct from its physiologic vasoconstrictive role. These indirect effects appear to be mediated by cytokines. Angiotensin II promotes the secretion of a number of inflammatory cytokines, including TGF-| , platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), IL-6, IL-12, TNF-a, and IFN-y.14-17 There are two receptors for angiotensin II, type 1 (AT1) and type 2 (AT2). AT1 receptors mediate the major vasoconstrictive activity of angiotensin II but also appear to be involved in angiotensin II-dependent augmentation of immune activation and stimulation of TGF-| production. The AT2 receptors are implicated in remodeling; may promote angiotensin II-dependent apoptosis; and have some functions that oppose the AT1 receptor, including vasodilation and increased production of nitric oxide.
Several of the genes encoding members of the renin-an-giotensin pathway exhibit polymorphisms that influence function. Genomic variants of the genes encoding AGT, ACE, AT1, and AT2 have been described.20 There is evidence that the AGT(A/A) and ACE(D/D) variants result in increased an-giotensin II activity; in turn, the angiotensin II can interact differentially with receptors of different genotypes and influence ultimate pathophysiology. A deletion variant of the ACE gene (D14091-14378) and a single-nucleotide polymorphic variant of the AGT gene (G ^ A, -6) are correlated with increased peripheral ACE activity and AGT levels, respectively.21,22 Both genotypes confer increased susceptibility to hypertension, and ACE(D14091-14378) also worsens ischemic heart disease and progression of intrinsic renal insufficiency [see Table 2].23 An analysis of ACE polymorphism in diabetes revealed that the ACE(D) allele is highly associated with diabetic nephropathy.
Of the different classes of white blood cells, T cells contain the highest level of ACE, approximately 28-fold more than
Figure 4 The renin-angiotensin system is illustrated, along with proven variants of genes responsible for its components. The variant genes indicated are thought to result in a quantitative increase in function in the system. The final hormone, angiotensin II (AII), has a variety of vasoactive, inflammatory, or anti-inflammatory effects, which appear to be dependent on the receptor that is engaged. (ACE— angiotensin-converting enzyme; AGT—angiotensinogen; AT1— angiotensin type 1 receptor; AT2—angiotensin type 2 receptor)
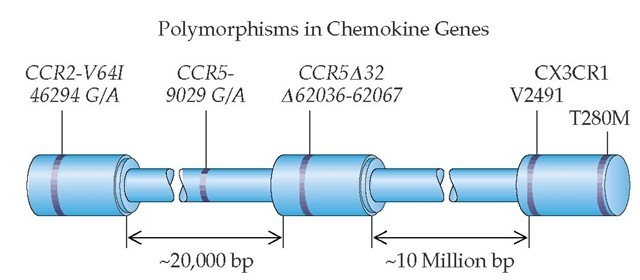
Figure 5 Locations of chemokine receptor genes and variant positions associated with those genes are shown. The CCR2, CCR5, and CX3CR1 genes are located on the same chromosome. CX3CR1 has two variable positions within the coding region. The location of the deletion variant of CCR5 and the location of the G-to-A variant of CCR5 in the 5′ promoter region of the gene are illustrated. The CCR2 gene has a G-to-A variant located within the coding region.
Indeed, immunologically competent T cells appear to be the major cell type expressing ACE in blood.25 The ACE expression can vary up to 100-fold during the differentiation of T cells. Monocytes express angiotensin II, the final product of the angiotensin synthetic pathway. Monocyte angiotensin II appears to mediate recruitment of inflammatory cells during renal damage through the synthesis of monocyte chemoattrac-tant protein-1.
A variant of the AT2 gene (A G, 1332) has been associated with congenital anomalies of the kidney and urinary tract. These developmental abnormalities are preceded by delayed apoptosis of undifferentiated mesenchymal cells surrounding the urinary tract during key ontogenic events.27 In kidney transplant recipients, specific variants of the ACE and AGT genes are correlated with poor clinical outcomes. Renal transplant patients who have either the ACE(D14091-14378) or the AGT homozygous (G ^ A, -6)/(G ^ A, -6) variant have poorer renal transplant function at 3 years, as well as more rapid progression of transplant failure, defined as an increase of serum creatinine levels over time. Diastolic blood pressure in these patients was also significantly higher as a function of the AT1(A ^ C, 1166) C gene dose. The pathophysiologic reasons for the association between specific angiotensin system gene polymorphisms and renal transplant outcomes are not well understood. Further work is needed to reveal the degree to which this association is a function of hypertensive organ damage or modulation of the im-munologic response mediated by angiotensin.
Chemokine receptor polymorphisms
Chemokines are molecules with a variety of functions, some of which influence the recruitment of inflammatory cells to sites of injury. Three of the genes encoding chemokine receptors are located on one chromosome. CCR2 and CCR5 are located within 20,000 base pairs of each other; CX3CR1 is located 10 million base pairs away from CCR5 [see Figure 5].
The leukocyte chemokine receptor CCR5 is expressed on monocytes, as well as on helper T cells involved in augmentation of the immune response (TH1 subset)28 [see 6:X Allergic Response]. CCR5 is a coreceptor for entry of HIV-1 into macro-phages. CCR5 binds the inflammatory chemokines RANTES (regulated on activation, normal T cell expressed and repeated), macrophage inflammatory protein (MIP)-1a, and MIP-1|, whereas CCR2 and CX3CR1 are receptors for the chemokines monocyte chemoattractant protein (MCP)-1 and fractalkine, respectively. Antagonists of CCR5, such as met-RANTES, prolong renal allograft survival in MHC-incompatible mice. Furthermore, prolonged heart transplant survival is achieved if the recipient is a homozygous CCR2 or CCR5 knockout. In humans, a 32-base-pair deletion of the CCR5 gene (CCR5 A32) renders the gene nonfunctional. There is also a polymorphic single-nucleotide variant of CCR5, CCR5-9029(G ^ A), which is located in the promoter region of the gene. The G variant is associated with defective transcription. Gene variants of some of these chemokine receptors have been associated with different rates of HIV disease progression.29 The CCR5 A32 variant is associated with lower incidence and severity of asthma and rheumatoid arthritis.30 Patients with homozygosity for CX3CR1-V2491I(G ^ A), CX3CR1-T280M(C ^ T), and CCR5-9029(G ^ A) have higher HIV progression rates. In contrast, patients with CCR2V64I(G ^ A) and CCR5(A32) exhibit slower progression of HIV, presumably because of reduced binding of the virus to target cells.
In renal transplant patients, the A/A homozygous genotype of the CCR59029(A ^ G) polymorphic locus is associated with significantly lower incidence of acute rejection episodes in the first posttransplant year. Although this could be explained by a protective effect of A/A homozygosity, it might instead be from a dominant detrimental effect of the G variant, given that both A/G heterozygotes and G/G homozygotes have been found to have similarly high rejection frequencies, which were twice that of patients with A/A genotype.
Practical Applications of Genotyping Polymorphisms
The genetic polymorphisms discussed in this subsection represent but examples of the many inherited variants of physiologically important genes that influence susceptibility to disease. These variants can act alone, in conjunction with variants at other loci, or through interaction with environmental factors to increase or decrease disease incidence or severity. The cy-tokine genes, chemokine genes, and genes of the renin-an-giotensin system are important modulators of the immune response and, in the case of the renin-angiotensin axis, of hypertension and vascular disease. Knowledge of a patient’s genotype may assist physicians in assessing prior risk of a pathophysiologic outcome and in tailoring therapy. Clinical trials may, in some cases, be better interpreted by knowledge of participant genotypes, because certain genotypes may impart differential incidence of disease and responsiveness to pharma-cologic agents.