General Considerations
Parasites are generally subdivided into three categories: protozoans, helminths, and arthropods such as ticks and insects. This topic focuses on helminthic pathogens; protozoans are discussed elsewhere, as are general characteristics of parasite-host relationships [see 7:XXX1VProtozoan Infections].
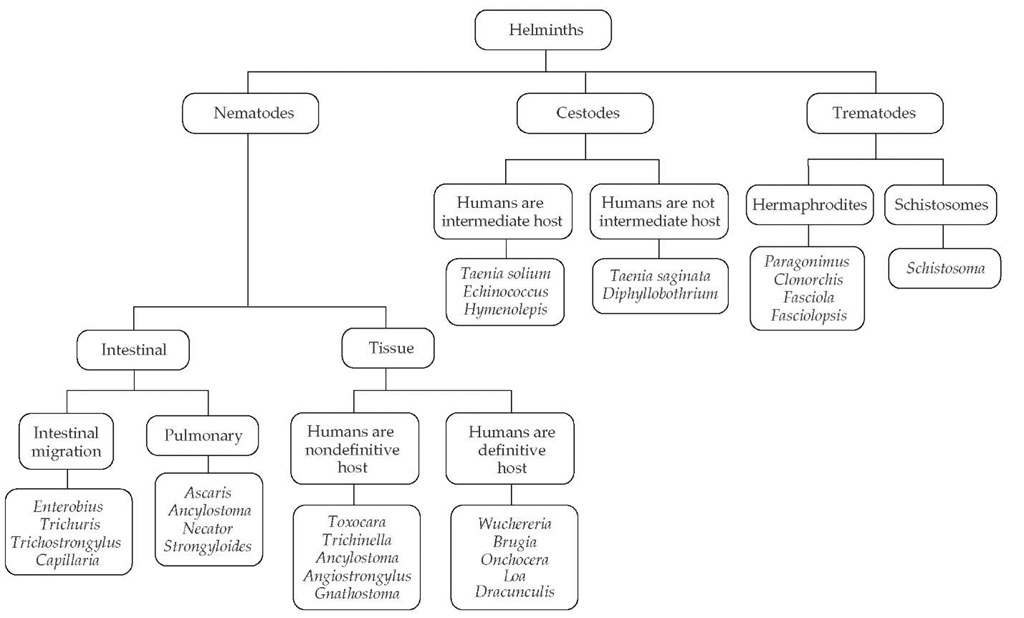
Helminthic parasites are divided into three major groups: nematodes (roundworms), cestodes (tapeworms), and trema-todes (flukes) [see Figure 1]. Helminthic parasites differ from protozoan parasites in several respects. First, protozoan parasites are unicellular organisms, whereas helminthic parasites are metazoan (multicellular) worms that possess differentiated organ systems. Second, most helminthic parasites do not replicate within the human host; rather, they develop to a certain stage within the human body and mature further outside the human body. During their extrahuman life cycle, helminths exist either as free-living organisms or as parasites within another host species and mature into new developmental stages capable of rein-fecting humans. Thus, with only a few exceptions (i.e., those parasites capable of internal reinfection, notably, Strongyloides sterco-ralis and Capillaria philippinensis), augmentation of the number of adult helminthic parasites that reside within the human host requires exogenous reinfection.
A third attribute of helminthic parasites but not of protozoan parasites is their tendency to elicit eosinophilia within the tissues and blood of infected humans. The magnitude of eosinophilia tends to correlate with the extent of tissue invasion by larvae or adult helminths. For example, in several helminthic infections, such as paragonimiasis, acute schistosomiasis, and Ascaris and hookworm infections, the elicited eosinophilia is greatest during the early phases of infection, when migrations of infecting larvae and the progression of subsequent developmental stages through the tissues are greatest. In established infections, local-tissue eosinophil infiltration is often present around helminths within tissues, but blood eosinophilia may be intermittent, mild, or absent. There may be no eosinophilia in established infections that are well contained within tissues (e.g., intact echinococcal cysts) or confined within the lumen of the intestinal tract (e.g., Ascaris and tapeworms). For some established infections, increases in blood eosinophilia may be episodic. Intermittent leakage of fluids from echinococcal cysts can elicit transient increases in blood eosinophil levels, as well as symptoms attributable to allergic or anaphylactic reactions (e.g., urticaria and bronchospasm).1 With tissue-dwelling helminths, increased eosinophilia may be associated with the migration of adult parasites, as in loiasis and gnathostomiasis.
Certain established infections—including trichinellosis, ani-sakiasis, gnathostomiasis, visceral larva migrans, echinococco-sis, and the several forms of filariasis—are capable of inducing eosinophilia but cannot be diagnosed on the basis of stool examination. In addition, some intestinal helminths may not be readily detectable on routine stool examination. The intestinal nematode that is most likely to cause persistent eosinophilia and that may not be detected on initial stool examination is S. stercoralis.2
Intestinal Nematode Infections
The major intestinal nematodes are roundworms, hookworms, whipworms, pinworms, and S. stercoralis [see Table 1 ]. Infection with these parasites occurs in many hundreds of millions of persons worldwide, especially in tropical areas. Children are particularly affected.
Roundworm, hookworm, and whipworm
Roundworms, hookworms, and whipworms are geohel-minths, requiring a soil phase for the fecally expelled eggs to develop into their infective stages. Thus, infections from these parasites usually occur in rural areas with poor sanitation. In these areas, highly prevalent infections with these intestinal nema-todes are major contributors to malnutrition in children.3
Epidemiology, Pathogenesis, and Clinical Features
Hookworm The World Health Organization (WHO) estimates that 700 million persons are infected with Ancylostoma duodenale or Necator americanus. Hookworm infection is more likely where the following conditions coexist: sanitary practices that permit human fecal contamination of the soil; soil that is damp enough for larval survival; and human contact with contaminated soil. Persons at risk include children, gardeners, plumbers or electricians in contact with soil, and infantry personnel.
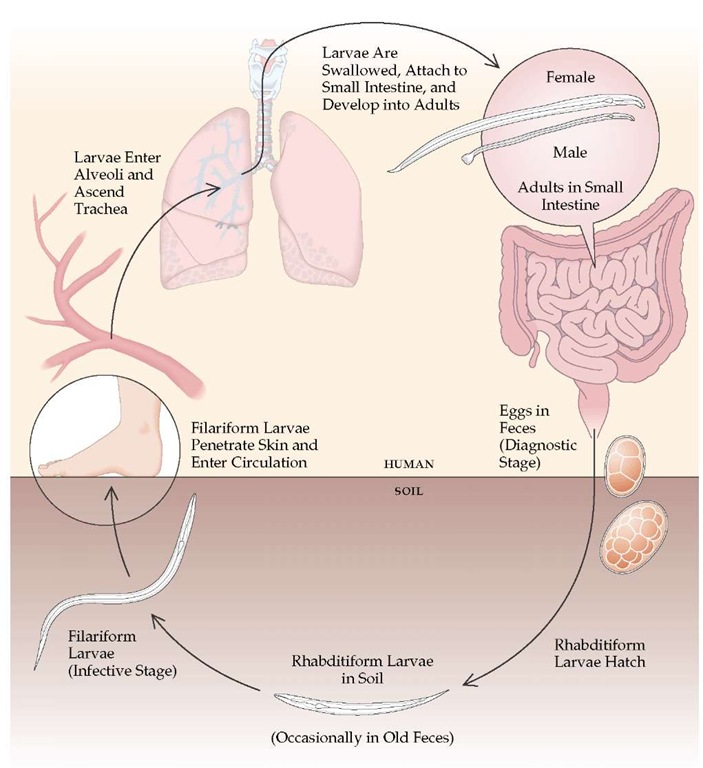
Hookworm eggs excreted in feces hatch in the soil, releasing larvae that develop into infective larvae [see Figure 2]. Percutaneous larval penetration is the principal mode of human infection, but infections with A. duodenale may also be acquired by oral ingestion. Larval penetration of the skin often produces a pruritic, maculopapular eruption at each site of entry. In persons previously infected, serpiginous tracts of intracutaneous larval migration, as in cutaneous larva migrans, can occur. From the skin, hookworm larvae travel via the bloodstream to the lungs. The hookworm larvae enter the alveoli, ascend the tracheo-bronchial tree to the pharynx, and are swallowed. The development of A. duodenale larvae can be arrested for many months before the larvae proceed to the lungs for subsequent maturation. Although transpulmonary larval passage may elicit a transient eosinophilic pneumonitis, this phenomenon is much less common with hookworm infections than with roundworm infec-tions.4 Larvae and young adult worms in the intestinal tract may cause gastrointestinal symptoms, including nausea, diarrhea, vomiting, abdominal pain (often with postprandial accentuation), and flatulence.
A major health impact of hookworm infection is iron loss resulting from the 0.1 to 0.4 ml of blood ingested daily by each adult worm [see Figure 3]. In malnourished hosts, such blood loss can lead to severe iron-deficiency anemia. The number of parasites necessary to cause anemia varies with host iron and protein stores, but hookworm burdens of 40 to 160 worms are associated with hemoglobin levels below 11 g/dl. Severe anemia from the interaction of malnutrition, malaria, and hookworm infection is a major contributor to childhood mortality in areas of the world where these conditions coexist.
Roundworm Ascaris lumbricoides is the most prevalent intestinal helminthic parasite, infecting well over one billion people worldwide. Each adult female can produce up to 200,000 eggs a day. Passed in feces, these eggs are remarkably resistant to environmental stresses and are capable of remaining viable for up to 6 years. With exposure to warm, humid soil, fertilized eggs become embryonated and infectious. Most transmission occurs by ingestion of fertilized eggs on dirty hands, in fecally contaminated agricultural products or other foodstuffs, or through geophagia (the life cycle of Ascaris is illustrated in the Centers for Disease Control and Prevention Public Health Image Library [CDC PHIL], at http://phil.cdc.gov/Phil; photograph 5231). Infection is more common in areas with poor sanitation. In regions with large concentrations of Ascaris eggs in the soil, eggs may be disseminated in the air, where they are inhaled and swallowed later with respiratory secretions.
Figure 1 Phylogenetic tree of the major helminths that afflict humans.
From swallowed Ascaris eggs, larvae hatch in the intestine within 1 to 2 days and molt into second-stage larvae, which are carried hematogenously to the liver and lungs. Roughly 1 to 2 weeks after infection, larvae penetrate alveoli from the capillary bed and molt into third-stage larvae, which ascend the tracheo-bronchial tree, are swallowed, and return to the intestine. These larvae mature into adult male and female worms, measuring 10 to 30 cm in length [see Figure 4]. The fertilized adult females begin producing eggs 2 to 3 months after the initial infection.
The clinical manifestations of ascariasis occur during early larval migration or with established intestinal infections. During the phase of transpulmonary migration, eosinophilic, often migratory, pulmonary infiltrates (Loffler syndrome) can develop, especially in previously sensitized hosts.4 The large adult worms in the intestine usually elicit no symptoms, although they may contribute to malnutrition.
Infrequently, adult Ascaris worms cause serious complications. In heavy infections, especially in children, the mass of entangled adult worms may cause partial or complete intestinal obstruction.5,6 A second type of complication can develop even when only a single adult worm migrates from its normal location within the intestinal lumen.Acute or recurrent migrations of one or more adult worms into the biliary tract can cause obstruction leading to acalculous cholecystitis, pyogenic cholangitis, pancreatitis, or liver abscesses.7 If the bowel wall is thinned or ulcerated by typhoid enteritis or other diseases, adult Ascaris worms may penetrate and perforate the intestine.
Heavy worm burden is more frequently seen in children younger than 10 years. Worm burden tends to decrease with increasing age, in correlation with increased secretion of cy-tokines by type 2 helper T cells (Th2) in response to Ascaris antigens.8 Thus, Th2 cytokine responses may protect against heavy infections.
Whipworm Infection with Trichuris trichiura is widespread in tropical regions throughout the world and also occurs in rural areas of the southern United States. Whipworm infection results from ingestion of eggs that have embryonated in the soil for 15 to 30 days (the life cycle of Trichuris is illustrated in the CDC PHIL [http://phil.cdc.gov/Phil], photograph 5231; a whipworm is shown in photograph 414).
Moderate whipworm infection provokes gastrointestinal complaints; heavy infections (> 200 worms) in children cause the Trichuris dysentery syndrome, characterized by chronic diarrhea, anemia, growth retardation, and, occasionally, rectal prolapse.
Laboratory Findings and Imaging Studies
Intestinal infections with roundworm, hookworm, or whip-worm are usually easily diagnosed by finding the eggs of the responsible parasite on stool examination. During the pulmonary phase of Ascaris or hookworm infections, the diagnosis is made by finding larvae in respiratory secretions. Results of stool examinations are usually negative during the pulmonary phase because this phase occurs early in infection, weeks or months before adult worms have matured sufficiently to liberate eggs into the feces. Positive stool samples during the respiratory phase indicate earlier long-standing infection. Thus, in eosinophilic pneumonitis, negative stool examination results do not exclude a parasitic etiology.
Table 1 Intestinal Nematodes
|
Roundworm (Ascaris lumbricoides) |
Pinworm (Enterobius vermicularis) |
Hookworm (Necator americanus or Ancylostoma duodenale) |
Whipworm (Trichuris trichiura) |
Strongyloides stercoralis |
|
|
Infective stage |
Egg |
Egg |
Larva |
Egg |
Filariform larva |
|
Route of infection |
Oral |
Oral |
Percutaneous Oral (A. duodenale) |
Oral |
Percutaneous or internally with ongoing auto-infection |
|
Extraintestinal penetration Larval stage |
After egg ingestion |
None |
During initial infection |
None |
During initial infection and during ongoing autoinfection |
|
Adult stage |
With aberrant migration |
Rarely via female genital tract |
None |
None |
None |
|
Adult length |
10-30 cm |
~ 1 cm |
0.6-1.2 cm |
3-5 cm |
~ 0.2 cm |
|
Principal symptoms |
Usually none; occasionally, GI or biliary tract obstruction by adult worms |
Perianal pruritus or none |
Usually none; iron deficiency anemia in heavy infection |
Usually none; GI symptoms or iron deficiency anemia in heavy infection |
In uncomplicated infection, none or GI symptoms and skin lesions; in disseminated disease, symptoms related to involvement of GI tract, lungs, or other organs, with possible bacteremia |
|
Diagnostic stage |
Eggs in stool |
Eggs from perianal skin on cellulose acetate tape |
Eggs in fresh stool; larvae in old stool |
Eggs in stool |
Rhabditiform larvae in stool; filariform larvae in duodenal aspirate, in pulmonary secretions, in stool, or in other fluids or tissues; serology helpful in low-grade infections |
|
Indications for therapy |
Presence of adult worms |
Symptoms |
Heavy worm burden; iron deficiency anemia |
Symptoms with heavy infection |
Presence of parasite |
|
Therapy (adult dose) |
Albendazole (400 mg p.o. once) or |
Pyrantel pamoate (11 mg/kg p.o. in a sin- |
Albendazole (400 mg |
Mebendazole (100 mg p.o., b.i.d., for 3 days or 500 mg |
Ivermectin (200 ^g/kg p.o., q.d., for 1-2 days) or |
|
Mebendazole (100 mg p.o., b.i.d., for 3 days or 500 mg p.o. once) or Ivermectin (150-200 ^g/kg p.o. once) or Nitazoxanide (500 mg p.o., b.i.d., for 3 days) |
gle dose; maximum: 1 g/day) or Mebendazole (100 mg p.o. in a single dose) or Albendazole (400 mg p.o. once) For each agent, repeat course after 2 wk |
p.o. once) or Mebendazole (100 mg p.o., b.i.d., for 3 days or 500 mg p.o. once) or Pyrantel pamoate (11 mg/kg p.o. in a single dose; maximum: 1 g/day) |
p.o. once) or Albendazole (400 mg p.o. for 3 days) or Ivermectin (200 ^g/kg p.o. daily for 3 days) or Nitazoxanide (500 mg p.o. daily for 3 days) |
Albendazole (400 mg p.o., b.i.d., for 2 days) or Thiabendazole (25 mg/kg p.o., b.i.d.; maximum dose, 3 g/day for 2 days or, in cases of disseminated disease, for 5 days) Immunocompromised persons may require prolonged therapy |
Adult Ascaris worms can be readily detected in upper GI series; the large worms are outlined by contrast material, and in late follow-up films, the parasite’s alimentary tract may be defined by a thin line of ingested contrast medium. Ultrasonogra-phy can detect adult worms in the small intestine, facilitating diagnosis of Ascaris as the cause of abdominal symptoms.10 Worms in the biliary tract can be detected by ultrasonography11 or endo-scopic cholangiopancreatography.12 At times, patients may note the passage of the large, smooth adult Ascaris worms in the stool or may cough up an adult worm.
Adult whipworms, which are 3 to 5 cm in length, may be visualized by anoscopy or colonoscopy. Adult hookworms, which are 0.6 to 1.2 cm in length, may be visualized by endoscopy of the proximal small intestine.
Treatment
Therapy for roundworm, hookworm, and whipworm infections utilizes the well-tolerated broad-spectrum anthelmintic agents mebendazole, albendazole, and, in some cases, pyrantel pamoate, ivermectin, and nitazoxanide [see Table 1]. Several caveats apply, however. Pyrantel is not used for whipworm or roundworm; ivermectin and nitazoxanide are not used for hookworm. Because mebendazole and albendazole may be terato-genic, they are contraindicated during pregnancy. Albendazole, ivermectin, and nitazoxanide are not yet approved by the Food and Drug Administration for these indications.
Figure 2 Hookworm infection typically begins when filariform larvae in soil penetrate the skin and enter the circulation. The larvae travel via the bloodstream to the lungs, where they cross into the alveolar air space and ascend the tracheobronchial tree. They are then swallowed and attach to the small intestine. There they develop into adults, which feed on blood. Adult females lay eggs that are excreted in feces and hatch in the soil, releasing rhabditiform larvae that develop into infective filariform larvae. The hookworm Ancylostoma duodenale may also be acquired by oral ingestion of larvae.
Therapy for Ascaris is mandatory to prevent unusual complications resulting from aberrant migration of the large adult worms. Adult Ascaris worms causing biliary and pancreatic obstruction may be removed at endoscopy.12
Anemia caused by hookworm responds to iron supplementation. Several animal models have shown that vaccination with recombinant hookworm antigens shows great promise in reducing the worm burden in humans and alleviating anemia.