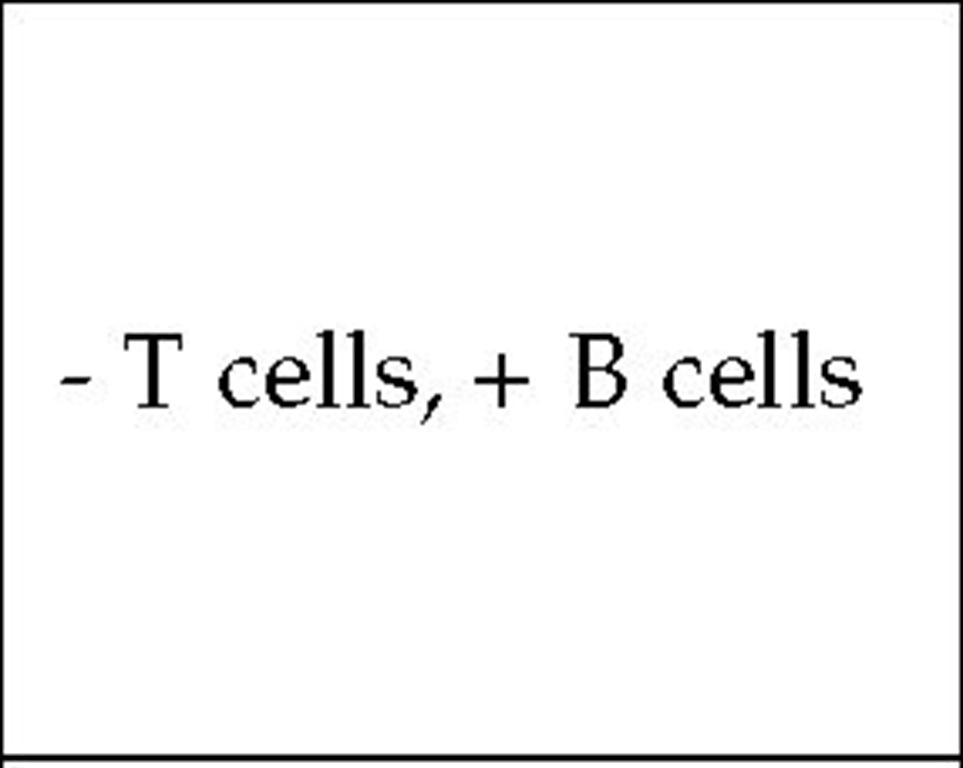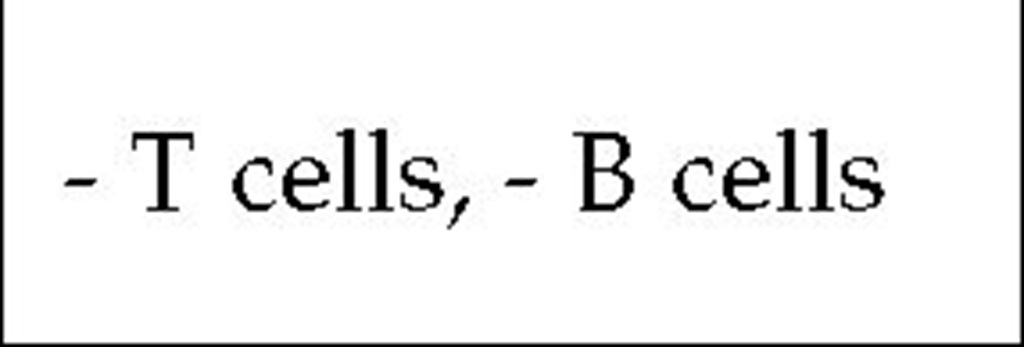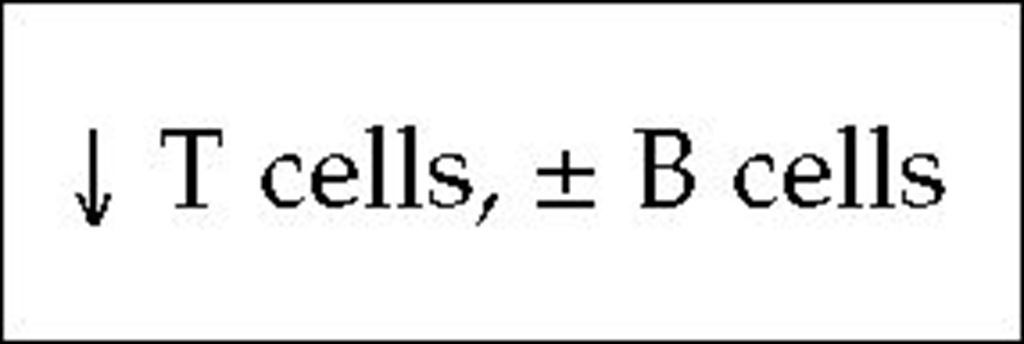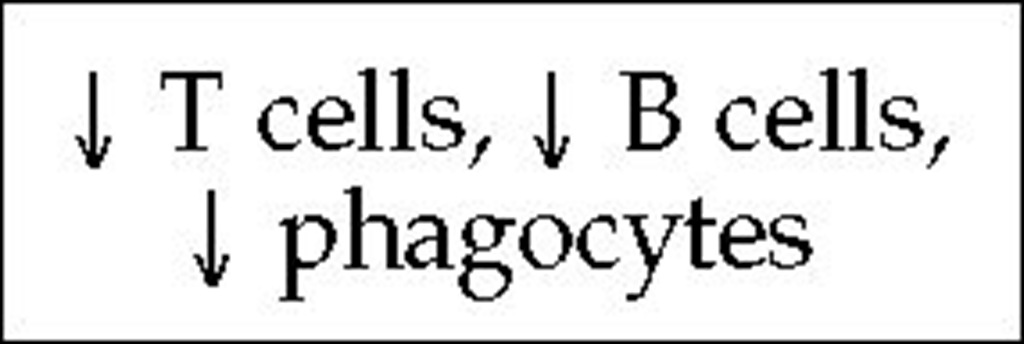Congenital thymic hypoplasia
Pathogenesis
Congenital thymic hypoplasia (DiGeorge syndrome) results from the lack of normal development of the third and fourth brachial, or pharyngeal, pouches, which leads to abnormality in the great vessels and to the absence of the thymus and the parathyroids. Congenital thymic hypoplasia is not inherited; rather, it is thought to result from an intrauterine accident occurring before the eighth week of pregnancy. The absence of the thymus leads to deficiency in cell-mediated immunity.
Diagnosis
Clinical manifestations Patients with congenital thymic hy-poplasia have distinctive facial features, including low-set ears, a shortened philtrum, and ocular hypertelorism. Hypocalcemia from associated parathyroid deficiency is a universal finding and often results in neonatal tetany. There can be a right-sided aortic arch or tetralogy of Fallot or many other cardiac malformations.
Laboratory tests The T cell defect in children with congenital thymic hypoplasia varies from mild to profound. Severely affected children do not exhibit delayed hypersensitivity reactions; their lymphocytes do not respond to mitogens or antigens in vitro, nor do they produce lymphokines. The lymph nodes lack paracortical lymphocytes. Plasma cells are present, however, and immunoglobulin levels are normal. Although patients with congenital thymic hypoplasia produce specific antibodies when they are immunized with various antigens, the antibody response is not quite normal, because secondary responses are lacking.
As the patient ages, T cell function improves; and usually by the time the child is 5 years of age, skin testing reveals no abnormality in cell-mediated immunity. However, the abnormal T cell phenotype—as indicated by a higher than normal ratio of CD4+ to CD8+ T cells—persists for life. Karyotyping reveals microdele-tions at chromosome 22q11 in approximately 90% of patients.
Treatment
Thymus transplantation should be undertaken in those infants with congenital thymic hypoplasia who experience frequent infections. Transplantation of fetal thymus results in rapid acquisition of normal T cell function, which is thought to be secondary to production of a thymic hormone secreted by the thymic epithelium. Rejection appears not to be a problem.
Table 3 Classification of Primary Specific Immunodeficiencies Involving Cell-Mediated Immunity
CMI—cell-mediated immunity
LSC—lymphocytic stem cell
HSC—hematopoietic stem cell
Severe combined immunodeficiency
Severe combined immunodeficiency disease (SCID) is characterized by marked depletion of cells that mediate both humoral and cellular immunity—B cells and T cells, respectively. SCID is fatal if left untreated.
Several variants of SCID have been identified. They are designated as T-B- or T-B+, depending on whether B cells are normal or increased (B+) or absent (B-). In addition to the extent of B cell involvement, the variants also differ in the site of the basic cellular defect, the pathogenetic mechanism, and the mode of inheritance [see Table 3].
Genetics and Pathogenesis
T-B+ SCID may be transmitted as either an X-linked or an au-tosomal recessive trait. The specific genetic defect responsible for the X-linked form of T-B+ SCID results from mutations in the y chain of the interleukin-2 receptor (IL-2R),20 whose gene is localized to the long arm of the X chromosome at Xq13.21 This y chain is also found in the receptors for IL-4, IL-7, IL-11, and IL-15.22 Engagement of the IL-7 receptor by IL-7 is required for T cell maturation, so precursor T cells in these patients do not mature.
When any of those receptors, or IL-2R, are engaged by its lig-ands, a cytoplasmic tyrosine kinase (Janus-family tyrosine kinase, or JAK3) bound to the y chain is activated. The gene encoding JAK3 is on an autosome, not the X chromosome. Thus, autosomal recessive T-B+ SCID is caused by mutations in the JAK3 gene.
T~B- SCID is inherited in an autosomal recessive manner. About half of the cases are caused by a deficiency in the enzyme adenosine deaminase (ADA),25 and another large fraction results from mutations in the recombination-activating genes RAG-1 and RAG-2 2 These recombinase enzymes are required for the gene rearrangements that occur before T cell receptor or immunoglobulin synthesis. Other patients with autosomal recessive T~B- SCID lack the enzyme purine nucleoside phosphorylase (PNP).
ADA deficiency leads to an accumulation of adenosine, adenosine triphosphate (ATP), and deoxy-ATP (dATP). It has been shown that dATP poisons ribonucleotide reductase, an enzyme required for DNA synthesis. Thus, lymphocytes lacking ADA cannot divide until the dATP overload is decreased or removed. In a similar manner, lymphocytes that lack PNP accumulate guanosine, guanosine triphosphate (GTP), and deoxy-GTP, causing metabolic abnormalities that resemble those seen in ADA deficiency. SCID caused by ADA or PNP deficiency can be diagnosed prenatally by amniocentesis because fibroblasts in the amniotic fluid also show the enzymatic defect.
|
Table 4 Laboratory Tests Used to Determine Deficiencies of Cell-Mediated Immunity |
|
Skin test: 24- to 48-hr reaction to Candida, Trichophyton, PPD |
|
Response to nonspecific mitogens: phytohemagglutinin, conca-navalin A, pokeweed mitogen |
|
Response to specific mitogens: diphtheria, tetanus, Candida |
|
Response to alloantigens: mixed lymphocyte reaction |
|
When responses to alloantigens and nonspecific and specific mitogens are negative: repeat tests while stimulating cells with IL-2 |
|
Enumerate T cells with monoclonal antibody to CD3, with or without a cell sorter |
|
Enumerate T cell subsets with monoclonal antibody to CD4 for helper T cells and with monoclonal antibody to CD8 |
|
Enumerate T cells positive for Ia (class II) antigens (which measures the number of activated T cells) |
|
Quantitate IL-2 receptors with monoclonal antibody TAC |
|
Quantitate IL-2 and interferon-gamma synthesis |
|
Enumerate NK cells with monoclonal antibodies Leu-7 and Leu-11 |
|
Assay NK cell activity using cell line K-562 |
|
Assay cytotoxic T cell activity using cell lines of cloned T cells |
|
Enumerate monocytes with monoclonal antibody Mo-1 |
|
Assay for IL-1 production by stimulated monocytes |
|
Determine serum level of anti-T cell antibodies |
|
Determine if antibody to HIV is present |
|
HLA typing |
|
Assay erythrocytes for adenosine deaminase and purine nucleo-side phosphorylase activity |
|
Detect thymus shadow on x-ray |
Note: All patients with defects in cell-mediated immunity should receive all tests listed, except the last three, for optimal examination. The last three tests are for patients suspected of having severe combined immunodeficiency or congenital thymic hypoplasia. HLA typing is needed for prospective recipients of bone marrow transplants.
IL-1—interleukin-1
IL-2—interleukin-2
PPD—purified protein derivative of tuberculin
NK—natural killer
CD8 deficiency is a rare form of SCID that results from mutations in the ZAP-70 gene.28,29 ZAP-70 is a tyrosine kinase that binds to the CD3 chain and is involved in signal transduction from the T cell receptor (the TCR-CD3 complex). CD8+ T cells fail to mature, and mature CD4+ T cells fail to function as a result of the mutations in ZAP-70.
Another variant of SCID is reticular dysgenesis, a severe combined immunodeficiency with a generalized granulocyte deficiency. Newborns with this disease lack granulocytes in the blood and bone marrow and die of infection within the first few days of life.
Diagnosis
Clinical manifestations Chronic pulmonary infections, diarrhea, moniliasis, and failure to thrive are the most common manifestations of SCID. The lymph nodes are small to absent despite chronic infections, which usually begin at 3 to 6 months of age.
Laboratory tests Complete blood counts show a low number of lymphocytes. There is absence of a thymic shadow on chest x-ray. (Autopsy in fatal cases has revealed an embryonic thymus that resembles the thymus at 6 weeks of gestation, before invasion with lymphocytes.) Tests for cutaneous delayed hypersensi-tivity and contact sensitization and in vitro assays of blood lymphocytes are negative, demonstrating the absence of T cells, a phytohemagglutinin response, and lymphokine production. Antibody levels are usually low, although occasionally the IgM level is normal; and sometimes, a myeloma component is seen.
Treatment
Hundreds of cases of SCID have been successfully treated by transplantation of bone marrow cells.30 By 3 to 8 months after receiving the bone marrow, these patients show normal delayed hypersensitivity and T cell function and are no longer abnormally susceptible to infection.
Immunologic reconstitution with bone marrow cells should be attempted only in specialized centers where comprehensive histocompatibility typing and intensive 24-hour care can be given. If the donor and the recipient are not exceedingly well matched, fatal graft versus host disease (GVHD) will ensue. Even an HLA-mismatched blood transfusion can produce fatal GVHD in such patients: the patient is immunocompromised and thus cannot reject the injected cells, but the histoincompatible cells that have been administered recognize the patient’s cells as foreign and react against them.
The manifestations of GVHD include fever, diarrhea, depression of the bone marrow, splenomegaly, and an erythematous rash on the face, trunk, and extremities. The reaction eventually leads to death. GVHD can be avoided by irradiating the blood before transfusion.
It is possible to establish grafts of half-matched (haploidenti-cal) parental marrow in infants with SCID. GVHD can be avoided in those cases if the parental marrow is depleted of T cells before transplantation by passage over lectin columns or by treatment with anti-T cell monoclonal antibody plus complement.
Patients with ADA deficiency have also been treated successfully with infusions of purified adenosine deaminase modified with polyethylene glycol. The ADA gene has been cloned and inserted into a retroviral vector.31 In a few ADA-deficient children, this vector has been transfected into peripheral blood lymphocytes, which were then reinfused. This gene therapy procedure has corrected the immunodeficiency in these patients, although it must be repeated periodically.32 Successful gene therapy has also been carried out in X-linked SCID by transducing a Maloney virus vector bearing the gene for the common y chain into bone marrow cells. Sustained responses have been reported in four of these patients: T cell number and function normalized in these patients, as did B cell function, and infusions of Y globulin were no longer required.
Wiskott-aldrich syndrome
An X-linked recessive disease, Wiskott-Aldrich syndrome (WAS), results from a mutation that has been mapped to the Xp11.3-p11.22 region of the X chromosome. The WAS gene has been cloned.
The lymphoid system of a patient with WAS appears anatomically intact at birth. Starting in the first months of life, however, there is a decrease in T cells in the paracortical areas of the lymph nodes and a polyclonal expansion of B cells. The T cells in these patients respond poorly to mitogens. The protein encoded by the WAS gene appears to be involved in signal transduction that leads to reorganization of the cytoskeleton when lymphocytes are stimulated, which results in defective collaboration between T cells and B cells. Lymphocytes have a markedly abnormal appearance when visualized by scanning electron microscopy. Platelets are abnormally small and few in number.36 Certain mis-sense mutations in the WAS gene lead to a mild disease called X-linked thrombocytopenia.
Diagnosis
Clinical manifestations WAS is characterized by eczema, easy bruising, increased susceptibility to infection (both pyogenic and opportunistic), and bloody diarrhea. These manifestations appear in the first months of life. An increased incidence of hematopoietic malignancies is seen, starting in the second or third decade of life.
Laboratory testing Patients with WAS have normal levels of IgG, high levels of IgE and IgA, and low levels of IgM. Severe thrombocytopenia is universal. Tests of cell-mediated immunity [see Table 4] show a variety of abnormalities: WAS patients lack isohemagglutinins and are unable to make antibodies to polysac-charides. They respond to some protein antigens but not to others; in addition, they may exhibit anergy and may not display positive results to skin tests for the usual bacterial or fungal antigens.
Treatment
WAS patients have been treated with marrow transplantation after receiving irradiation or busulfan and antilymphocyte serum to destroy residual lymphocytes; they have then shown normal immune and platelet functions. In WAS patients who do not receive a bone marrow transplant and who experience severe bleeding from thrombocytopenia, splenectomy may be considerably beneficial.
Immunologic deficiency with ataxia-telangiectasia
Ataxia-telangiectasia (A-T) is a disease associated with defects in cell-mediated immunity and with immunoglobulin deficiencies. It is inherited as an autosomal recessive trait. The gene for A-T (ATM for A-T mutated) maps to the chromosomal region 11q22.3.39,40 Normally, the gene appears to function in repair of breaks in double-stranded DNA. Patients with A-T have a disorder of the cell-cycle checkpoint pathway that results in an extreme hypersensitivity to ionizing radiation. Consequently, frequent chromosomal breaks, inversions, and translocations are observed. Postmortem examination may disclose abnormalities in the thymus, which is small and deficient in lymphocytes. There also may be an abnormality in lymph node structure.
Diagnosis
Clinical manifestations A-T presents as a progressive neurologic disease that begins in early childhood. It is characterized by cerebellar ataxia, starting at 18 months of age, followed by increasing tremor and deterioration of mental function. By 5 years of age, progressive telangiectasia is seen in the vessels of the bul-bar conjunctiva and is later visible on the skin. The immune deficiencies in these patients leads to recurrent sinus and bronchial infections and subsequent bronchiectasis. An unusually high incidence of lymphoid malignant disorders has been reported in patients with A-T.41
Laboratory testing About 70% of patients with A-T have a severe deficiency in IgA. On tests of cell-mediated immunity [see Table 4], some A-T patients are anergic and fail to show delayed hypersensitivity responses to common microbial antigens. They may also have abnormal in vitro cell-mediated immune responses and may tolerate allografts.
Treatment and Prognosis
No satisfactory treatment for A-T is currently available. Persons with A-T who survive into their second decade may fail to mature sexually. A-T patients usually die of lymphoid malignancies or other causes by the end of their second decade.