Gene Therapy
been widely used because of their capacity to integrate into
INTRODUCTION
a host chromosome and potentially express foreign proteins
indefinitely.
Molecular genetic studies during the last decades have
A sampling of expression systems and their uses is given
led to an enormous increase in our understanding of the
here to illustrate the approaches that are being followed. Every
molecular biology of the replication of viruses. The com-
virus system has advantages and disadvantages as a vector,
plete nucleotide sequences of many virus genomes have
depending on its intended use. One of the more exciting uses
been determined. Information on the origins required for
has been the development of viruses as vectors for gene ther-
the replication of these genomes, the promoters used to
apy, that is, to correct genetic defects in humans. In the most
express the information within them, and the packaging
general sense, gene therapy involves transfer of genetic infor-
signals required for packaging progeny genomes into viri-
mation into a cell, tissue, organ, or organism with the goal of
ons have been established for many. The mechanisms by
improving the clinical outcome, either by curing a disease,
which viral mRNAs are preferentially translated have been
or alleviating an underlying condition in a patient. Although
explored. Together with methods for cloning and manipu-
results have been disappointingly slow in coming, such sys-
lating viral genomes, this information has made possible
tems offer great promise. This use represents an example of
the use of viruses as vectors to express foreign genes. In
taking these infectious agents that have been the source of
principle, any virus can be used as a vector, and systems
much human misery and developing them for the betterment
that use a very wide spectrum of virus vectors have been
of mankind. Such expression systems have a wide variety
described. DNA viruses were first developed as vectors,
of other potential uses, however. Efforts to engineer viruses
since it is possible to manipulate the entire genome in the
to kill cancer cells are also receiving attention. Viruses that
case of smaller viruses, or to use homologous recombina-
express foreign proteins have potential uses in the engineer-
tion to insert a gene of interest in the case of larger viruses.
ing of new vaccines against other pathogens. Finally, viral
Recent developments now make it possible to manipulate
expression systems have proved very useful in the expression
the entire genomes of even very large DNA viruses as artifi-
of proteins in cell culture that can be used for various studies.
cial chromosomes, which potentially makes the use of these
large genomes for expression of foreign proteins even more
appealing. When complete cDNA clones of RNA viruses
VIRUS VECTOR SYSTEMS
were obtained, it became straightforward to rescue plus-
strand viruses from clones because the viral RNA itself is
A representative sampling of viruses that are being devel-
infectious, and many such viruses have been used to express
oped as vectors is described next in order to illustrate some
proteins. The use of minus-strand RNA viruses as vectors
of the strengths and weaknesses of the different systems.
was delayed because the virion RNA itself is not infectious,
The viruses used in most clinical trials to date have been the
but recent developments has made it possible to rescue virus
poxviruses, the adenoviruses, and the retroviruses, and these
from cloned DNA by using coexpression of the appropriate
are described here. Several other virus systems that may be
viral proteins in a transfected cell. As a consequence, minus-
used in the future for treatment of humans, or that are useful
strand RNA viruses have also joined the club of expression
for other purposes, are also described.
systems receiving intense study. Retroviruses have also
virulence. A new approach to the use of poxvirus vectors
Vaccinia Virus
has been the development of nonhuman poxviruses, such as
Vaccinia virus is a poxvirus with a large dsDNA genome
canarypox virus, as vectors. Canarypox virus infection of
of 200 kb (Chapter 7). Until recently, this genome was too big
mammals is abortive and essentially asymptomatic, but for-
to handle in one piece in a convenient fashion, and homolo-
eign genes incorporated into the canarypox virus genome are
gous recombination has been used to insert foreign genes into
expressed in amounts that are sufficient to obtain an immu-
it. The large size of the viral genome, however, does mean
nologic response.
that very large pieces of foreign DNA can be inserted, while
A variety of approaches have been used to obtain recom-
leaving the virus competent for independent replication and
binant vaccinia viruses that express a gene of interest, but
assembly. Another advantage of the virus is that it has been
only the first such method to be used, and one that remains
used to vaccinate hundreds of millions of humans against
in wide use, is described here. This method is illustrated
smallpox. Thus, there is much experience with the effects of
in Fig. 11.1. The thymidine kinase (TK) gene of vaccinia
the virus in humans. Although the vaccine virus did cause
virus is nonessential for growth of the virus in tissue culture.
serious side effects in a small fraction of vaccinees, highly
Furthermore, deletion of the TK gene results in attenuation
attenuated strains of vaccinia have been developed for use
of the virus in humans, which is a desirable trait. Finally,
in gene therapy by deleting specific genes associated with
the TK gene can be either positively or negatively selected
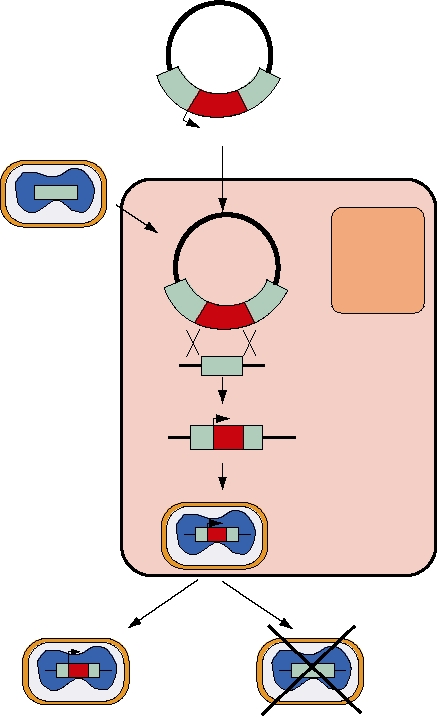
Bacterial
Plasmid
TK gene-flanking
sequences and
FG
foreign gene (FG)
wt vaccinia virus
TK
Nucleus
FG
Homologous
recombination
FG
Virus production
Recombinant
vaccinia virus
BUdR selection
TK-
TK+
FIGURE 11.1 Construction of recombinant vaccinia virus expression vector. The foreign gene (red) is inserted into
a bacterial plasmid adjacent to a vaccinia promoter (black arrow), flanked with sequences from the vaccinia thymidine
kinase gene (turquoise). Plasmid DNA is transfected into cells infected with wild-type (TK+) vaccinia virus. Recombinant
progeny from homologous recombination are all TK-, due to interruption of the TK gene, and can be selected by growth
in bromodeoxyuridine (BUdR), since incorporation of BUdR into TK+ vaccinia is lethal. These TK- vaccinia will infect
normally and express the foreign gene under control of the vaccinia promoter. Adapted from Strauss and Strauss (1997)
Figure 2.25 on p. 115.
by using different media for propagation of the virus.
Baculoviruses
The starting point is a plasmid clone that contains a copy
The baculoviruses are insect viruses that have a large
of the TK gene that has a large internal deletion. In the
DNA genome capable of accommodating large DNA inserts.
region of the deletion a vaccinia virus promoter is inserted
Foreign DNA is inserted by recombination and selection of
upstream of a polylinker. The gene of interest is inserted
appropriate viruses. They have been widely used to express
into the polylinker using standard cloning technology.
high levels of protein in eukaryotic cells (insect cells in this
Thus, we have the foreign gene downstream of a vaccinia
case) that can be used, for example, in crystallization trials to
promoter, and the entire insert is flanked by sequences from
determine protein structure, or to produce protein for immu-
the vaccinia TK gene. The plasmid containing the cloned
nization of animals, and other uses. Recent studies have sug-
TK gene with its foreign gene insert is transfected into
gested that baculoviruses might be useful for gene therapy
cells that have been infected by wild-type vaccinia virus.
in humans. The viruses will infect a number of human cells
Homologous recombination between the TK gene in the
resulting in expression of proteins of interest, but the viruses
virus and the TK-flanking sequences in the plasmid occurs
are nonpathogenic in humans, suggesting a level of safety
with a sufficiently high frequency that a reasonable frac-
in their possible application. Whether problems associated
tion of the progeny have the gene of interest incorporated.
with these viruses, such as low levels of expression and the
These viruses have an inactive TK gene (they are TK-),
rejection of them by the immune system, can be overcome
because the TK gene has been replaced by the deleted ver-
remains to be determined.
sion containing the inserted foreign gene. The next step,
then, is to select for viruses that are TK- by growing virus
in the presence of bromodeoxyuridine (BUdR). An active
Adenoviruses
TK enzyme will phosphorylate BUdR to the monophos-
Adenovirus infections of humans are common and nor-
phate form, which can be further phosphorylated by cel-
mally cause only mild symptoms. Deletion of virulence
lular enzymes to the triphosphate and incorporated into
genes from adenovirus vectors further attenuates these
the viral nucleic acid during replication. Incorporation of
viruses. In addition, adenovirus vaccines have been used by
BUdR is lethal under the appropriate conditions, and thus
the military for some years and, therefore, some experience
viruses that survive this treatment are those in which the
has been gained in the experimental infection of humans
TK gene has been inactivated.
by adenoviruses, although gene therapy trials use a differ-
It is usually necessary to select among the surviving prog-
ent mode of delivery of adenovirus vectors. Because of their
eny for those that possess the gene of interest, because inac-
apparent safety, adenoviruses have been developed for use
tivation of the TK gene can occur spontaneously through
as vectors in gene therapy trials or for vaccine purposes.
deletion or mutation. Selection can be accomplished by a
Two approaches have been used. In one, infectious adenovi-
plaque lift hybridization assay in which virus in plaques is
ruses have been produced that express a gene of interest. In
transferred to filter paper. Virus plaques on the filter paper are
the second approach, suicide vectors are produced that can
probed with radiolabeled hybridization probes specific for the
infect a cell and express the gene of interest, but which are
inserted gene. Virus in plaques that hybridize to the probe is
defective and cannot produce progeny virus. Suicide vec-
recovered and further passaged. In this way a pure virus stock
tors cannot spread to neighboring cells, and the infection is
that will express the gene of interest can be isolated.
therefore limited in scope and in duration.
The genome of adenoviruses is dsDNA of 36 kbp (Chapter
Herpesviruses
7). Thus, the genome is smaller than that of poxviruses
or other large DNA viruses such as the herpesviruses and
The herpesviruses also have a large DNA genome that
the baculoviruses and can accommodate correspondingly
is capable of accommodating large inserts of foreign DNA.
smaller inserts. However, inserts large enough for most
HSV-1, in particular, has been studied as an expression vec-
applications can be accommodated. The genome is small
tor. Recombination has been used to insert foreign genes
enough that the virus can be reconstituted from DNA clones.
and to delete virus genes involved in lytic growth or toxic-
Such an approach is inconvenient, however, and homolo-
ity. Because HSV-1 is neurotropic, it has been considered
gous recombination is often used to insert the gene of inter-
as a possible vector for the control or eradication of neural
est into the virus genome.
cancers. The viral DNA does not integrate, and the virus is
The foreign gene is inserted into the region occupied by
capable of infecting nonreplicating neurons and being main-
either the adenovirus E1 or E3 genes, one or both of which
tained in a latent state, properties that suggest it could be
are deleted in the vector construct. Virus lacking E1 can-
used for this purpose. It might also be used as an expression
not replicate, and such viruses form suicide vectors. For
vector that could produce protein for long periods in neu-
gene therapy, suicide vectors are normally used so as to
rons, and as such might be useful for the treatment of spinal
prevent the spread of the infection. To prepare the stock
nerve injury, for example, or for pain therapy.
of virus lacking E1, the virus must be grown in a cell line
is formed. The stock of defective virus must be tested to
that expresses E1. An overview of this process is shown
ensure that no replication-competent virus is present, since
in Fig. 11.2. The complementing cell line, which produces
such virus can arise by recombination between the vector
E1 constitutively, supplies the E1 needed for replication
and the E1 gene in the complementing cell line.
of the defective adenovirus. The cells are transfected with
Adenoviruses with only E3 deleted are often used to
the defective adenovirus DNA and a full yield of progeny
express proteins for vaccine purposes. These E3-deleted
virions results. The progeny virus is defective and cannot
viruses possess intact E1 and will replicate in cultured
replicate in normal cells, but it can be amplified by infection
cells and in humans, but are attenuated. Because the virus
of the complementing cell line. On introduction of the virus
replicates, expression of the immunizing antigen persists
into a human, the virus will infect cells and express the
for a long time and a good immune response usually
foreign gene, but the infection is abortive and no progeny virus
results.
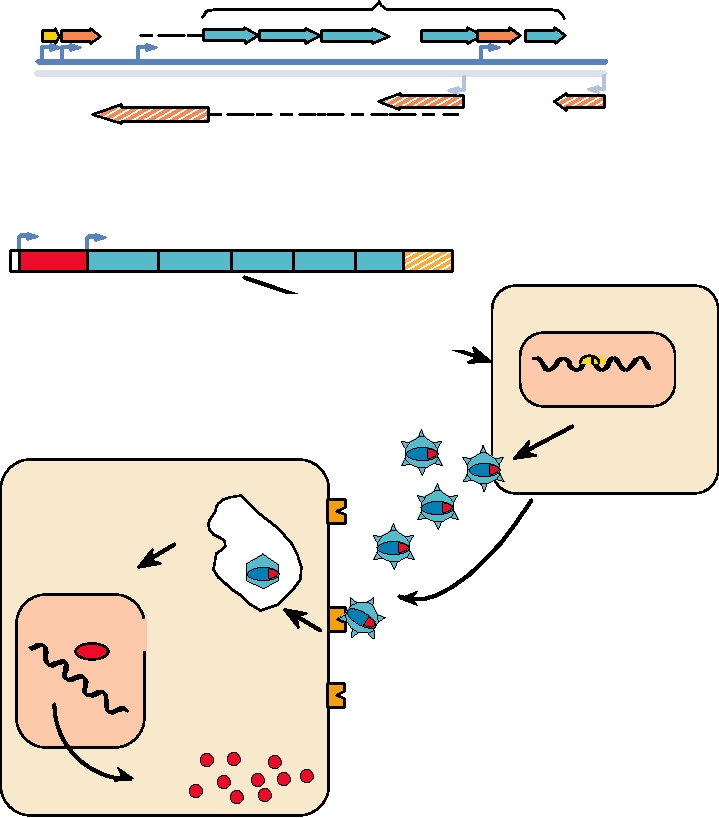
A. Wild-type Adenovirus Genome
Virion Structural Proteins
E1A E1B
L1
L2
L3
L4
L5
E3
E
R
DNA
L
E
E4
2B
2A
B. Adenovirus vector DNA (E1, E3 deleted, expression cassette inserted)
Expression
Transfect adenovirus vector DNA
cassette
Complementing Cell Line
into complementing cell line
that expresses the E1A gene
E1
Endosome
Target Cell
Vector DNA packaged into
virion particles
Infect target cell
Epichromosomal
action
Product of expression
cassette
FIGURE 11.2 Generation of a nonreplicating adenovirus expression vector. From the wild-type adenovirus genome,
the E1 and E3 genes are removed. The E1 genes are replaced with an expression cassette. This adenovirus DNA is
transfected into a complementing cell line that produces E1 protein. The transfection produces particles that are able to
infect cells, but which are E1 and nonreplicating. The DNA genome is delivered to the nucleus where it functions as an
epichromosome and directs expression of the inserted foreign gene. Adapted from Crystal (1995).
The procedure for insertion of the gene of interest by
genes. In the process, all of the essential cis-acting signals
homologous recombination resembles that used for the pox-
required for packaging, reverse transcription, and integra-
viruses. The gene is inserted into a plasmid containing flank-
tion are retained. The foreign gene can be under the control
ing sequences from the E1 or E3 region, and transfected
of the LTRs, or it can be under the control of another pro-
into cells infected with adenovirus. Recombinant viruses
moter positioned in the insert upstream of it. The resulting
containing the gene of interest are selected and stocks pre-
DNA clone is transfected into the packaging cell line, and
pared. It is also possible to transfect cells with the E1 or E3
a producer cell line isolated that expresses the vector DNA as
expression cassette together with DNA clones encoding the
well as the helper DNA. Vector RNA transcribed from the
rest of the adenovirus genome, in which case homologous
vector DNA is packaged into retroviral particles, using the
recombination results in the production of virus. In the case
proteins expressed from the helper DNA. These particles are
of insertions into E1, cells that express E1 must be used to
infectious and can be used to infect other cells or to transfer
produce the recombinant virus.
genes into a human. On infection of cells by the packaged
vector, the vector RNA is reverse transcribed into DNA that
integrates into the host-cell chromosome, where it can be
Adeno-Associated Viruses
expressed under the control of the promoters that it contains.
Adeno-associated viruses (AAVs) have a single-stranded
The limitation on the size of the insert is about 10 kb, the
DNA genome of 4.7 kb (Chapter 7). They normally require
upper limit of RNA size that can be packaged.
coinfection of a cell by a helper virus, usually an adenovi-
Although murine leukemia viruses are not known to cause
rus or a herpesvirus. They are being developed as expres-
disease in man, it has been found that these viruses will cause
sion vectors because they are not pathogenic in humans and
tumors in immunosuppressed subhuman primates. Thus, it is
because they normally integrate into the host-cell genome in
thought to be essential that there be no replication-competent
a specific region, thus minimizing the problems of insertional
virus in stocks used to treat humans. Replication-competent
mutagenesis. The genome size is small enough to be readily
virus can arise during packaging of the vector by recombi-
manipulated as a DNA clone, but the small size also limits the
nation between the vector and the retroviral sequences used
amount of DNA that can be inserted and therefore the appli-
to produce GagPolEnv. At the current time, preparations
cability of the virus for gene transfer experiments. A related
of packaged vectors are screened to ensure that replication-
problem is that for expression studies, the genome is normally
competent viruses are not present. Efforts are being made to
deleted for the AAV genes with only the ends that function
reduce the incidence of recombination during packaging in
as promoter sites retained. However, site-specific integration
order to simplify the procedure. One approach is to develop
requires the activity of the Rep protein. Nonetheless, the sys-
vectors that have very little sequence in common with the
tem is sufficiently attractive that efforts to develop AAV as a
helper sequences, in order to reduce the incidence of homol-
gene therapy vector continue and the virus has been used in a
ogous recombination. A second approach is to separate the
number of clinical trials, as described later.
GagPol sequences from the Env sequences in the helper
cell. In this case, recombination between three separate DNA
fragments in the producer cell (that encoding GagPol, that
Retroviruses
encoding Env, and sequences in the vector) are required in
Retrovirus-based expression systems offer great promise
order to give rise to replication-competent retrovirus.
because the retroviral genome integrates into the host-cell
In gene therapy trials that use retroviruses, it has been
chromosome during infection and, in the case of the simple
found that the expression of the foreign gene in humans is
retroviruses at least, remains there as a Mendelian gene that
often downregulated after a period of months. Attempts are
is passed on to progeny cells on cell division. Thus, there is
being made to identify promoters that will not be downregu-
the potential for permanent expression of the inserted gene of
lated. Different promoters might be required for different
interest. The essential components of a retrovirus vector are the
uses, and promoters that target transcription to particular cell
long terminal repeats (LTRs), the packaging sequences known
types would be useful.
as ψ, the primer-binding site, and the sequences required for
A major problem with retroviral vectors is that simple ret-
jumping by the reverse transcriptase during reverse transcrip-
roviruses will only infect dividing cells. Although they enter
tion to form the dsDNA copy of the genome (Chapter 6).
cells and are reverse transcribed into DNA, the DNA copy of
The process of creating and packaging a retrovirus-based
the genome can enter the nucleus only during cell division.
expression cassette is illustrated in Fig. 11.3. A packaging
In many gene therapy treatments, it is desirable to infect stem
cell line is created that expresses the retroviral gag, pol, and
cells in order to maintain expression of the therapeutic gene
env genes, but whose mRNAs do not contain the packag-
indefinitely. Because stem cells divide relatively infrequently,
ing signal and so cannot be packaged. The vector DNA/
it is difficult to infect a high proportion of them by vectors
RNA is created by modifying a DNA clone of a retrovirus
used to date. Attempts are being made to identify methods
to contain the gene of interest in place of the gagpolenv
to stimulate stem cells to divide during ex vivo treatment,
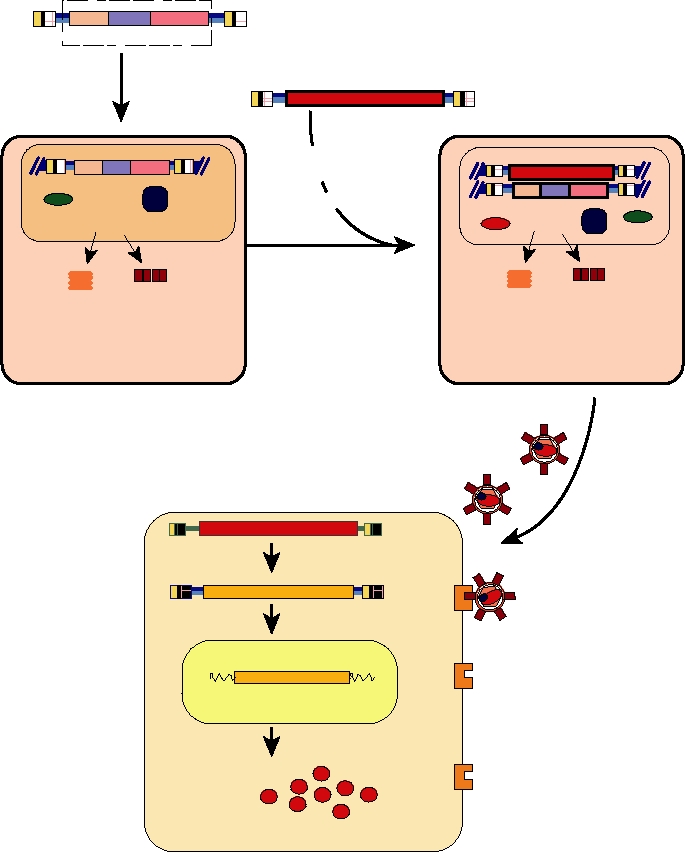
Mammalian-type C Retrovirus DNA lacking ψ
pol
env
LTR
gag
LTR
Retrovirus vector DNA
ψ
A
Integration
Expression cassette
B Transfect packaging
ψ
cell line
Expression cassette
Integration
ψ
wt RNA
RT
wt RNA
Vector RNA
RT
Glycoproteins
Capsid Glycoproteins
Capsid
C
Producer Cell Line
Packaging Cell Line
D Infect target cell
with packaged
vector
Expression cassette
vector
RNA
Reverse Transcription
E
proviral
vector
Expression cassette
DNA
F
Random integration
Expression Products
G
Target Cell
FIGURE 11.3 Scheme for producing a packaged replication-defective retrovirus expression vector. (A) A "packaging"
cell line is generated by introduction of DNA encoding gag, pol, and env genes into the chromatin of a fibroblast cell
line. This DNA lacks the packaging signal Ψ and RNA transcribed from it is not packaged. (B) The packaging cell
line is transfected with a second retroviral DNA, in which the foreign gene (expression cassette) replaces gag, pol, and
env, but which has an intact Ψ packaging signal and intact LTRs, to form a "producer" cell line. This producer cell line
(C) releases vector particles containing the expression cassette genome packaged with the proteins from the helper
genome. (D) These particles enter target cells via specific cell surface receptors, (E) are reverse transcribed, (F) randomly
integrate, and (G) produce expression products. Adapted from Dunbar (1996).
so that a larger fraction of them can be infected. A second
directed against an antigen expressed only on the target
approach is to develop lentivirus vectors. Lentiviruses, which
cells. In principle, this approach is feasible, but whether it
include HIV, can infect nonreplicating cells and could poten-
can be developed into something practical is as yet an open
tially infect nondividing stem cells during ex vivo treatment.
question. If specific cells could be infected, it would allow
Lentivirus vectors could also be useful for therapy involving
protocols in which the therapeutic gene would be expressed
other nondividing cells, such as neurons.
only in cells where it would be most useful. It would also
It would be of considerable utility to be able to target ret-
allow the specific killing of cells such as tumor cells or HIV-
roviruses to specific cells. One possible approach to this is
infected cells. For example, the retrovirus could express a
to replace all or part of the external domains of the retroviral
gene that rendered the cell sensitive to toxic drugs such as
surface glycoprotein with a monoclonal antibody that is
BUdR. A retrovirus vector that expressed such a gene could
also be useful for conventional gene therapy, because it
one for the structural proteins and the second for the gene of
would allow the infected cells to be killed if the infection
interest. The size of the insert must be relatively small, on the
process threatened to get out of hand.
order of 2000 nucleotides or less, because longer RNAs are
not packaged efficiently. However, this system has the advan-
tage that the resulting double subgenomic virus is an infectious
Alphaviruses
virus that can be propagated and maintained without helpers.
The genomes of plus-strand RNA viruses are self-
A second approach is to delete the viral structural pro-
replicating molecules that replicate in the cytoplasm, and
teins and replace them with the gene of interest. In this
they can express very high levels of protein. These properties
case, there is room for an insert of about 5 kb that will still
make them potentially valuable as expression vectors.
allow the resulting replicon to be packaged. The replicon
The alphaviruses possess a genome of single-strand RNA of
is capable of independent replication, and transcription of a
about 12 kb (Chapter 3). Their genomes can be easily manipu-
subgenomic messenger results in expression of the gene of
lated as cDNA clones, and infectious RNA can be transcribed
interest. The replicon constitutes a suicide vector. It cannot
from these clones by RNA polymerases, either in vivo or in
be packaged unless the cells are coinfected with a helper to
vitro. RNA transcribed in vitro can be transfected into cells
supply the structural proteins, or unless a packaging cell line
and give rise to a full yield of virus, whereas RNA transcribed
that expresses the viral structural proteins is used.
in vivo will begin to replicate and produce virus. The structural
Alphavirus replicons can be extremely efficient in
proteins are made from a subgenomic mRNA, making it easy
expressing a foreign gene. In some cases as much as 25% of
to insert a foreign gene under the control of the subgenomic
the protein of a cell can be converted to the foreign protein
promoter. Two approaches that have been used are illustrated
expressed by the replicon over a period of about 72 hours.
in Fig. 11.4. In one approach, a second subgenomic promoter is
Wild-type replicons are cytolytic in vertebrate cells, inducing
inserted into the genome downstream of the structural proteins,
apoptosis, and the infection dies out. However, replicons
or between the structural proteins and the nonstructural pro-
have been produced with mutations in the replicase proteins
teins (Fig. 11.4C). Two subgenomic mRNAs are transcribed,
that are not cytolytic and will produce the protein of interest
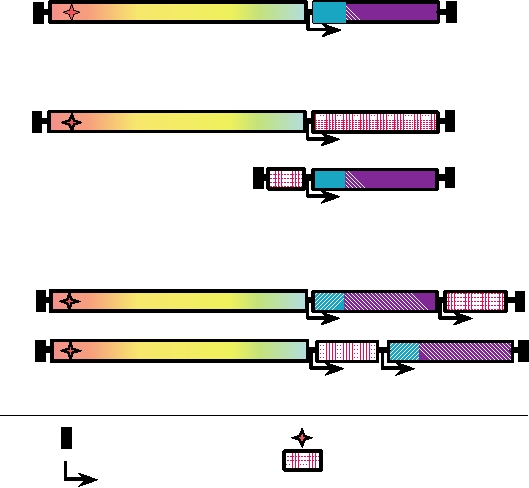
A. Alphavirus genome organization
Replicase proteins
Structural proteins
An
CAP
SG
B. Alphavirus replicon packaged
with a nonpackaged helper
An
Replicase
CAP
SG
An
CAP
Structural proteins
SG
C. Nondefective alphavirus expression vectors
with two subgenomic promoters
Structural proteins
CAP
Replicase
An
SG
SG
An
CAP
Replicase
Structural proteins
SG
SG
Encapsidation signal
Replication promoter
Foreign gene
Subgenomic promoter
SG
FIGURE 11.4
Alphavirus expression vectors. (A) The genome organization of a typical alphavirus with the location
of the promoters for replication and production of subgenomic RNA as well as the RNA-packaging signal indicated.
(B) A simple alphavirus replicon. The structural proteins of the virus have been replaced with the foreign gene to be
expressed. If packaging of the replicon is required, the structural proteins of the virus are supplied on a DI RNA lacking a
packaging signal. (C) Packaged expression vectors with two subgenomic promoters. These constructs are unstable if the
foreign gene is much larger than 2 kb. Adapted from Strauss and Strauss (1994) Figure 23.
indefinitely. Thus, a wide sprectrum of choices is available,
and produces the subgenomic mRNA that is translated into the
and the system chosen can be adapted to the needs of a par-
gene of interest. As described in Chapter 10, naked DNA can be
ticular experiment or treatment.
used to transfect muscle cells and perhaps other cells.
Viral expression systems would be more useful if they
could be directed to specific cell types. An approach that uses
Polioviruses
monoclonal antibodies to direct Sindbis virus to specific cells
has been described. Protein A, produced by Staphylococcus
Plus-strand viruses that do not produce subgenomic
aureas, binds with high affinity to IgG. It is an important com-
mRNAs, such as the picornaviruses and flaviviruses, present
ponent of the virulence of the bacterium because it interferes
different problems for development as vectors. The trans-
with the host immune system. The IgG-binding domain of pro-
lated product from the gene of interest must either be incor-
tein A has been inserted into one of the viral glycoproteins.
porated into the polyprotein produced by the virus and
Virions containing this domain are unable to infect cells using
provisions made for its excision, or tricks must be used to
the normal receptor. However, the virus will bind IgG mono-
express the gene of interest independently. Two approaches
clonal antibodies. If an antibody directed against a cell surface
with poliovirus will be described as examples of how such
component is bound, the virus will infect cells expressing this
viruses might be used as vectors.
protein at the cell surface. Thus, this system has the potential
Poliovirus replicons have been constructed by deleting
to direct the virus to a specific cell type. One of the advantages
the region encoding the structural proteins and replacing this
of this approach is that the virus, once made, can be used with
sequence with that for a foreign gene. The foreign gene must be
many different antibodies and thus directed against a variety of
in phase with the remainder of the poliovirus polyprotein, and
cell types. This approach is potentially applicable to any envel-
the cleavage site recognized by the viral 2A protease is used
oped virus, and perhaps to nonenveloped viruses as well.
to excise the foreign protein from the polyprotein. Because the
A modification of the alphavirus system is to use a DNA
poliovirus replicon lacks a full complement of the structural
construct containing the replicon downstream of a promoter for
genes (it is a suicide vector), packaging to produce particles
a cellular RNA polymerase, rather than using packaged RNA
requires infection of a cell that expresses the polioviral struc-
replicons. On transfection of a cell with the DNA, the replicon
tural proteins by some mechanism. A construct that uses this
RNA is launched when it is transcribed from the DNA by cellu-
approach to express the cytokine tumor necrosis factor alpha
(TNF-α) is illustrated in Fig. 11.5. A poliovirus "infectious
lar enzymes. Once produced, the RNA replicates independently
A. Poliovirus infectious clone
P1
P2
P3
T7
SalI
EcoRI
3B
5 NTR
Poly (A)
4
3
2
1
2A 2B
2C
3A 3C
3D
(60nt)
B. Poliovirus replicon encoding TNF-α
P1
P2
P3
EcoRI T7
SalI
3B
5 NTR
Poly (A)
TNF-α 2A 2B
4
2
2C
3A 3C
3D
(60nt)
T-Y
G-V-D-L-R
V-N-T-K-D-L-T-T- Y
G
2Apro
2Apro
Coding domains
Exogeneous gene to be expressed
Nonstructural proteins
Vpg
Structural proteins
FIGURE 11.5 Generation of poliovirus replicons for expression of foreign genes in motor neurons. Based on an earlier
construct to express interleukin-2 via a poliovirus replicon, the gene for wild-type murine tumor necrosis factor alpha
(TNF-α) was positioned between the VP0 and 2A proteins of poliovirus, replacing VP3 and VP1. It was flanked on either
side by sites for cleavage by the poliovirus 2A protease. These constructs were injected into transgenic mice expressing
the poliovirus receptor, and expression of murine TNF-α was monitored. Adapted from Bledsoe et al. (2000).
clone" in which a DNA copy of the viral genome is positioned
a cassette comprised of the gene of interest preceded by the
downstream of a promoter for T7 RNA polymerase is modified
specific intergenic sequence of the parental virus.
by replacing the genes for VP3 and VP1 with the gene for TNF-
The generation of a recombinant MHV (mouse hepatitis
α. Recognition sites for the poliovirus 2A protease are posi-
virus) encoding Renilla luciferase is shown in Figure 11.6 to
tioned on both sides of the TNF-α gene. The TNF-α protein
illustrate the strategies employed. These include maintenance
is produced as part of the poliovirus polyprotein, and cleaved
of a replication defective genome as a bacterial plasmid under
from the polyprotein by the 2A protease. Packaged replicons
the control of a T7 promoter, transcription of RNA in vitro,
were used to infect transgenic mice that expressed the polio
electroporation of RNA into feline cells previously infected
receptor (Chapter 1). One of the interests of this system is that
with a murine coronavirus engineered to infect feline cells, in
poliovirus exhibits an extraordinary tropism for motor neurons
vivo recombination, and selection for recombinants on murine
in the central nervous system (CNS) (Chapter 3). The packaged
cells. Due to the high frequency of recombination in coronavi-
replicons, on introduction into the CNS, infected only motor
ruses, many recombinants are unstable and not suitable for use
neurons, and therefore the foreign gene was expressed only in
as vaccines. However, the location of the foreign gene within
motor neurons. Such replicons may be useful to treat CNS dis-
the genome, the particular coronavirus used, and the identity
eases in which motor neurons are affected.
of the particular heterologous gene all have significant effects
A second approach to the use of poliovirus replicons is to
on stability, and coronaviruses may yet prove to be useful for
use a second internal ribosome entry site (IRES) (Chapter 1)
targeted gene delivery.
to initiate the synthesis of the nonstructural proteins. If the
foreign gene replaces the structural genes, it will be translated
from the 5′ end of the genome. If the poliovirus nonstructural
Rhabdoviruses and Other Negative
genes are placed downstream of a second IRES, internal ini-
Strand Viruses
tiation at this IRES results in production of a polyprotein for
In minus-strand RNA viruses, the genomic RNA is not
the nonstructural proteins. This approach is similar to the
itself infectious. Ribonucleoprotein containing the N, P, and
approach shown in Fig. 3.3, where the structural proteins are
L genes is required for replication of the viral RNA, and thus
replaced by a gene of interest.
for infectivity, and only recently have methods been devised
to recover virus from cDNA clones. A schematic diagram of
how virus can be recovered from DNA clones of the rhabdovi-
Coronaviruses as Expression Vectors and
rus vesicular stomatitis virus (VSV) (Chapter 4) is shown in
Vaccine Candidates
Fig. 11.7. A cell is transfected with a set of cDNA clones that
The coronaviruses have long been considered as potential
together express N, P, and L as well as the genomic or antig-
candidates for experimental expression vectors or as candi-
enomic RNA. The antigenomic RNA usually works better,
date vaccines. The viruses have the largest nonsegmented
probably because it does not hybridize to the mRNAs being
RNA viral genomes, up to 31 kb (see also Chapter 3), which
produced from the plasmids. Encapsidation of the antige-
is both an advantage in that they could potentially accommo-
nomic RNA by N, P, and L to form nucleocapsids allows it to
date a large amount of heterologous nucleic acid, and a dis-
replicate and produce genomic RNA that is also encapsidated.
advantage due to the difficulties of manipulating large RNA
Synthesis of mRNAs from the genomic RNA, together with
molecules. The essential genes are arranged 5′-replicase-S-E-
continued replication, results in a complete virus replication
M-N-3′, and are interspersed with a number of nonessential
cycle and production of infectious progeny virus that have as
genes which are group specific. Recent studies have revealed
their genome the RNA supplied as a cDNA clone. The yield
a number of characteristics which make them even more
of infectious virus is small, but sufficient to isolate individual
attractive as vector candidates. The first was that the deletion
plaques and thus obtain viruses from the cDNA clones.
of the nonessential genes is sufficiently attenuating that
The ability to rescue virus from a cDNA clone makes it
no further mutations in the essential genes are required to
possible to manipulate the viral genome. Since the rhabdovi-
produce an avirulent virus. Second, as the precise domains
rus genome is transcribed into multiple mRNAs, one for each
on the S protein which interact with the species-dependent
gene, and the transcription signals recognized by the enzyme
cellular receptors were determined, it was found that both
are well understood, it is relatively simple to add or delete
species and tissue specificity could be altered by relatively
genes. A modified VSV that was produced by using DNA
minor changes in the sequence of S. Third, it is possible to
clones is illustrated in Fig. 11.8. In this VSV, the surface
rearrange the linear order of the genes, and while this altered
glycoprotein present on the VSV particle, called G, has been
the relative amounts of the products, it reduces the possibil-
deleted and replaced with CD4, the cell surface protein that
ity that the vector (vaccine) could undergo recombination
is used as a receptor by HIV. In addition a new gene has been
with field strains. Fourth, it is possible to insert heterolo-
inserted, the gene encoding the HIV coreceptor CXCR4, so
gous genes anywhere in the genome by simply incorporating
that the virus now contains six genes. The virions produced
Search WWH :