to completely eradicate the virus in these areas is due to the
as well as the adaptive system and has been described. Other
difficulty of immunizing infants with a live virus vaccine
components of the innate system include the cytokines, a
while maternal antibodies are still protective. In regions of
complicated set of small proteins that are powerful regula-
Africa, measles is so widely epidemic that many infants con-
tors of the immune system. Many cytokines are critical for
tract the disease as soon as the protection due to maternal
the function of the adaptive immune system, as described
antibodies wears off. Multiple, spaced immunizations for
in part before. Cytokines are also important components of
each infant would be required to successfully immunize the
the innate system. The innate system also includes natural
population of new susceptibles before the epidemic virus
killer cells, cytotoxic lymphocytes that kill cells that do not
gets there, which is difficult to achieve in countries with
express adequate levels of class I MHC molecules. Apoptosis
limited health care facilities. In an effort to get around this
is another innate defense mechanism, because most cells are
problem, very young infants were immunized with higher
programmed to die if they sense that they are infected.
doses of the live virus vaccine in a trial in Senegal, on the
theory that using a larger dose would overcome the effects of
Toll-Like Receptors
maternally derived immunity. This trial was a failure, how-
ever, because for unknown reasons the cumulative mortality
Initiation of an immune response requires the recognition
in infants receiving the high titer vaccine was greater than
of "pathogen-associated molecular patterns" or PAMPs for
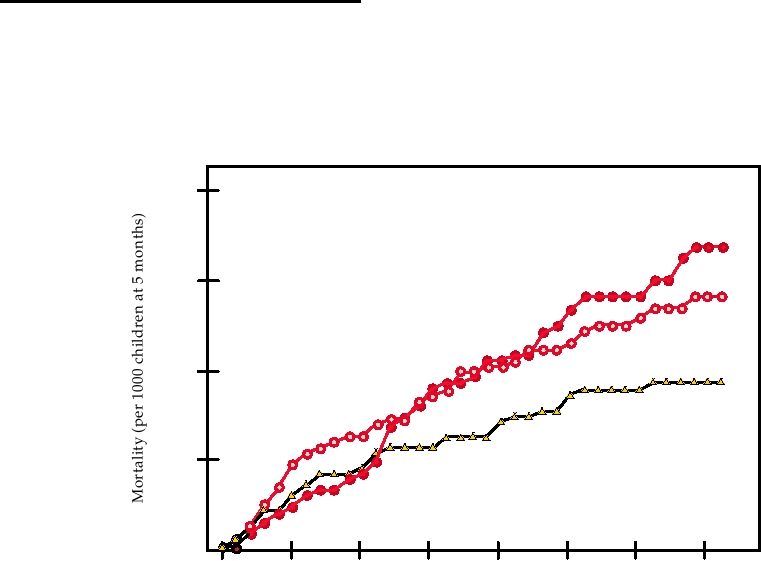
that in controls (Fig. 10.14). Thus at present it is very diffi-
short, which are conserved molecular motifs present in or
cult to devise methods that will effectively immunize young
produced by invading pathogens but which are not present
infants in these countries against measles, but programs are
in their hosts. The sensors that recognize PAMPs are called
being developed to multiply immunize infants at different
pattern recognition receptors or PRRs. The best known
ages using national immunization days in order to overcome
PRRs are Toll-like receptors (TLRs) but other PRRs exist,
these problems.
such as the protein called RIG-1 described later.
TLRs are components of ancient pathways of innate
immunity to invading microbes. They recognize a number
INNATE IMMUNE SYSTEM
of conserved structures in microbes and upon activation
lead to the production of antimicrobial peptides or to activa-
The innate immune system is composed of a large number
tion of components of the innate and the adaptive immune
of elements that attempt to control infection by pathogens,
systems. The Toll pathway was first discovered in the fruit
but this system is independent of the identity of the particular
fly, Drosophila melanogaster. Activation of this pathway
pathogen. Complement is a component of the innate system
in Drosophila results in the production of peptides that
200
EZ-HT
150
SW-HT
Standard
100
50
0
40
20
35
25
30
10
15
5
Age (months)
FIGURE 10.14 Child mortality after high-titer measles vaccination in Senegal. Three groups of children were given
either 105.4 PFU of Edmonston-Zagreb vaccine at 5 months of age (EZ-HT), or 105.4 PFU Schwartz vaccine (SW-HT) at
5 months of age or the standard schedule (placebo at 5 months and 103.7 PFU vaccine at 10 months of age.) Adapted from
Garenne et al. (1991).
render the fly resistant to fungal infection and to infection
intracellular compartments, usually thought to be endosomal
by Gram-positive bacteria. Flies that lack this pathway are
compartments. Examples of TLRs activated by nonself mol-
very sensitive to infection by fungi or Gram-positive bacte-
ecules include TLR4, activated by exposure to lipopolysac-
ria. Resistance of flies to Gram-negative bacteria is medi-
charide (LPS), a component of the cell wall of gram-negative
ated by another pathway called the IMD pathway. Innate
bacteria, as well as by other molecules, including the enve-
defenses against microorganisms are also known to exist in
lope proteins of some viruses; TLR5, activated by exposure
starfish, nematodes, plants, and virtually all other organisms
to bacterial flagellin; and TLR2, activated by components
that have been examined, and many of these defense mecha-
from Gram-negative bacteria, mycoplasma, spirochetes, and
nisms are known to rely on Toll-like proteins.
trypanosomes, among others. TLRs activated by exposure to
Mammalian homologues of Toll have been described
nucleic acids include TLR9, activated by exposure to non-
only recently. There are at least 11 TLRs in humans that can
methylated CpG in DNA, which is rare in mammalian DNA
be grouped into five subfamilies. They are integral mem-
(CpG itself is underrepresented in mammalian DNA and it
brane proteins, some of which are expressed at the cell sur-
is usually methylated) but common in bacteria and in DNA
face and some of which are present in intracellular vesicles.
viruses; TLR3, activated by double-stranded RNA, which
They possess extracytoplasmic leucine-rich domains that are
is produced by most viruses after infection; and TLR7 and
important for recognition of the molecular stimuli and intra-
TLR8, which recognize single-stranded RNA. Overall, then,
cytoplasmic domains that signal, via various intermediates,
a wide variety of molecules produced by invading viruses,
to start production of effector proteins. A partial list of the
bacteria, fungi, and other pathogens is recognized by various
ligands that stimulate various TLRs is shown in Table 10.4.
TLRs and an immune response is generated. Of interest also
Some TLRs recognize nonself molecules as is the case for
is the fact that TLR4 responds to heat shock proteins (HSP60
TLR1, 2, 4, 5, and 6, whereas others recognize nucleic acids
and HSP70), which are produced by the host in response to
in unfamiliar contexts as in the case for TLR3, 7, 8, and 9.
stress, which can include the stress induced by infection.
In general, TLRs in the first class are expressed at the cell
TLRs that are expressed on the cell surface respond to
surface whereas TLRs of the second class are expressed in
extracellular stimuli, whether present there because of cell
TABLE 10.4
Characterization of Toll-Like Receptors
Toll-like receptor
Major cell types
Ligands
Source of ligand
TLRs found predominantly in the cell plasmalemma
TLR1/TLR2 heterodimer
Monocytes, mDCs
Tri-acyl lipopeptides
Bacteria/mycobacteria
GP-1 anchored proteins
Parasites
TLR2/TLR6 heterodimer
Monocytes, mDCs, pDCs
Di-acyl lipopeptides,
Bacteria/mycobacteria,
lipoteichoic acid,
gram-positive bacteria
Zymosan
Fungi
TLR4 (homodimer)
Monocytes, differentiated DCs
LPS
Gram-negative bacteria
Taxol
Plant
HRSV fusion protein
Paramyxoviridae
Heat shock proteins
Mammalian host
Fibrinogen
Mammalian host
Envelope proteins
MMTV (Retroviridae)
TLR 5
Monocytes
Flagellin
Motile bacteria
TLRs found predominantly in intracellular compartments
TLR3
mDCs (also surface of fibroblasts)
dsRNA
Many viruses; Reoviridae
TLR7
???
ssRNA, some siRNAs
Many viruses including: HIV (Retroviridae),
VSV (Rhabdoviridae), Influenza
TLR8
Monocytes, mDCs
ssRNA
Many viruses; Rhabdoviridae
TLR9
pDCs
CpG DNA
Bacteria
CpG DNA
DNA viruses, esp. Herpesviridae
Abbreviations: mDCs, myeloid dendritic cells; pDCs, plasmacytoid dendritic cells; HRSV, human respiratory syncytial virus; MMTV, mouse mammary
tumor virus. Interactions most important for controlling viral infection are shown in red.
Source: Data from Takeda et al. (2003); O'Neill (2005); Iwasaki and Medzhitov (2004); Kawai and Akira (2006).
death that releases these components or because extracellu-
have a net positive charge, and are produced by cleavage
of propeptides. They fall into three subfamilies called α-,
lar microorganisms are present. TLRs in intracellular com-
β-, and θ-defensins. α- and β-defensins are linear polypep-
partments may also be responding to extracellular molecules
tides whereas θ-defensins are cyclic 18mers. Only α- and
that enter the endosomal compartment by endocytosis, or
β-defensins are produced in humans. They are mainly pro-
they may be intracellular molecules that enter the intracel-
lular compartments where TLRs are present during infec-
duced by leukocytes and epithelial cells.
α- and β-defensins have diverged from a common
tion or replication. Many (most?) TLRs function as dimers.
β-defensin ancestral gene that is expressed as far back in
Heterodimers of TLR1 and TLR2 and of TLR6 and TLR2
have been found, and these two heterodimers have different
evolution as snakes. There are multiple genes for them. Six
human α-defensins have been identified, and gene-based
specificities. TLR4 is commonly found as a homodimer.
searches indicate that there are about 30 β-defensin genes
Various TLRs are expressed in most tissues of the body.
Particularly noteworthy is the expression of multiple TLRs
in humans and more than this in mice. These polypeptides
by monocytes, macrophages, dendritic cells, and mast cells,
will kill a number of microbes in vitro, and model studies in
key players in the immune system. Activation of TLRs
mice have shown that they are important in protecting mice
leads to activation of transcription factors such as NFκB
from bacteria that infect the lungs or the gut. Production of
α-defensins is constituitive for the most part, whereas pro-
that results in the production of a number of end products.
duction of β-defensins is usually inducible. Induction can be
In some cases, antimicrobial peptides are produced, includ-
ing in humans various defensins described later. Of equal
through the activation of Toll-like receptors or through the
or greater importance, however, is the expression of proin-
activity of cytokines. Defensins appear to work by permeabi-
flammatory genes such as TNF-α, IL-6, IL-1β, and IL-12 in
lizing the microbial membrane, but they also appear to recruit
response to activation of several TLRs. Of particular impor-
other elements of the immune system to the site of infection.
tance, type I interferons are produced in response to activa-
They may have evolved to fight off bacteria, fungi, and proto-
tion of TLR3, 4, 7, 8, and 9. The inflammatory response that
zoa, but studies have shown that they are also effective against
results from the activation of TLRs is crucial for the activa-
at least some viruses. Their antiviral activity is of two types.
tion of other components of the innate system as well as for
They can directly inactivate some enveloped viruses, probably
the activation of the adaptive immune system. In particular,
in the same way that they inactivate bacteria. They can also
type I IFNs are key players in starting and organizing an
interact with potential target cells to interfere with virus rep-
immune response. TLR-signaling also leads to the matura-
lication by mechanisms that are poorly understood, and these
tion of dendritic cells, key players in the adaptive immune
mechanisms are potentially effective against both enveloped
and nonenveloped viruses. It is noteworthy that both α- and
system. Activation of dendritic cells may be direct, by infec-
β-defensins have been found in breast milk, suggesting that
tion of these cells by viruses, for example, or indirect, as
the result of signaling by other infected cells. There appear
they are important for protecting the infant from infection.
to be multiple kinds of dendritic cells that express different
combinations of TLRs. Upon activation of a dendritic cell, it
Natural Killer Cells
migrates to lymph nodes where it presents antigens to T cells
and stimulates T cells that recognize the antigen presented by
Natural killer (NK) cells are cytolytic cells that kill by an
it to become active. Thus, the maturation of dendritic cells
antigen-independent mechanism. They are important for the
induced by activation of TLRs is critical for the activation of
control of many virus infections, as shown by the fact that
the adaptive immune response. Overall, therefore, TLRs are
ablation of NK cells leads to more serious disease. NK cell
not only important as the first line of defense against invad-
activity increases within the first 23 days after infection by
ing microorganisms via the production of antimicrobial pep-
a virus, stimulated by the presence of interferon and per-
tides or the induction of cytokine expression that slow their
haps by other cytokines, and thereafter the number of cells
growth and in some cases eradicate the infection, but also
declines. One of the functions of these cells is to kill cells
for activation of an adaptive immune response to eliminate
that do not express class I MHC or that express it in only
the invading pathogen and cause the organism to become
low amounts. Such cells, of course, do not present antigen
immune.
to CTLs and therefore escape normal immune surveillance.
NK cells express two sets of receptors on their surface. One
set of receptors interacts with MHC class I molecules on the
Defensins
surface of the target cell. This interaction inhibits killing by
Organisms from plants to mammals produce antimicrobial
the NK cell. The second set of NK receptors interacts with
peptides that are important in defending the organism from
activating molecules on the surface of cells. Interaction with
infection. Mammals produce two classes of such peptides,
activating molecules will stimulate the NK cell to kill the
called cathelicidins and defensins. Defensins are peptides
target cell if the NK cell is not sufficiently inhibited by its
of 18 to 45 amino acids that possess three disulfide bonds,
interaction with class I molecules.
Many different viruses downregulate the production of
cells that are no longer needed, cells that are damaged in
MHC in infected cells in order to escape immune surveil-
some way (such as by exposure to UV light or to free radi-
lance. The elimination of these infected cells by NK cells is
cals), or cells that are dangerous to the host because they are
of considerable importance to the host. It seems clear that
infected or because their cell cycle is deregulated (and they
NK cells evolved to rid the body of cells that are not subject
are therefore potential tumor cells). Cells that are no longer
to normal immune surveillance, which could include tumor
needed include excess cells that are produced during devel-
cells as well as virus-infected cells that no longer express
opment and must be eliminated (a normal occurrence during
adequate levels of MHC. In the one case known of a human
development) and T cells responsible for the control of an
with a deficiency in NK cells, as well as in experimental stud-
infectious agent after the infection has been eliminated (and
ies with mice in which NK cells are depleted, infection by
which may even be dangerous if left around). Thus, apopto-
a number of viruses that downregulate production of MHC
sis serves many roles in an organism and the basic machin-
results in a much more severe disease than that produced in
ery has been conserved throughout evolution. In mammals it
individuals able to mount a normal NK response.
serves as a component of both the adaptive immune response
(ridding the body of unneeded lymphocytes and killing of
infected cells by CTLs) and the innate immune response
Apoptosis
(suicide by infected cells).
Apoptosis or programmed cell death is a defense of last
Several different mechanisms for the induction of apop-
resort by an infected cell. It is a cell suicide pathway in which
tosis exist, as would be expected from the many functions
mitochondria cease to function, chromatin in the nucleus
served by apoptosis. Furthermore, the control of apoptosis is
condenses, nuclear DNA is degraded, and the cell fragments
very complicated, which is necessary to control an event as
into smaller membrane-bound vesicles that are phagocy-
drastic as cell death. Some of the pathways used to induce
tosed by neighboring cells. Apoptosis serves to eliminate
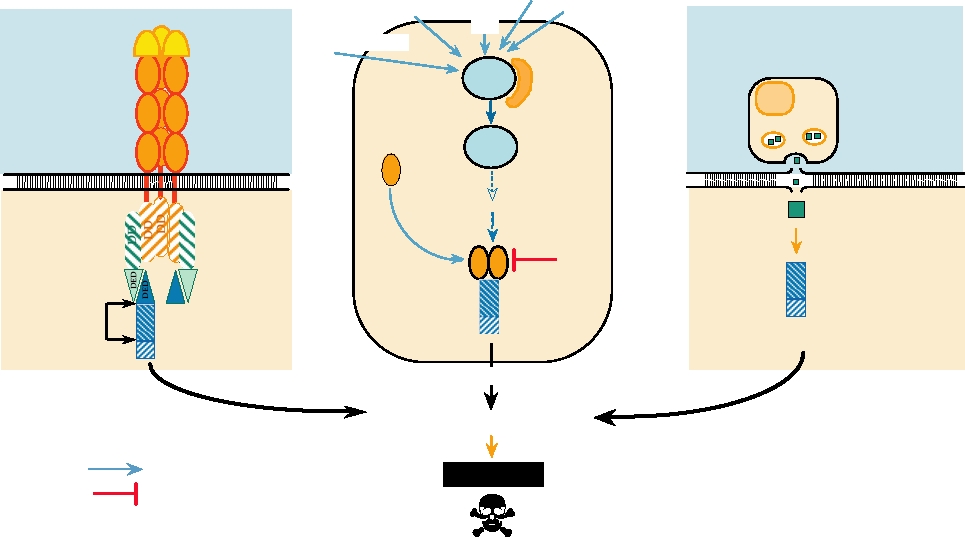
apoptosis are illustrated in Fig. 10.15. One pathway involves
Killling via Receptor
Killing due to External Stimuli
Killing by CTL
UV irradiation
via the Granzyme B Pathway
Unscheduled
Hypoxia
DNA synthesis
E2F
Viral infection
Ligand
FASL
p53
Mdm2
CTL
Receptor
Fas
p53
Perforin Channel
Bax
Granzyme
??
Cytoplasmic
death domains
FADD
Bcl-2
Death effector
domains
Procaspase
Procaspase
Activation
Procaspase
cleavages
Caspase cascade
Caspase cascade
Caspase cascade
Effector caspases
Induction
Apoptosis
Inhibition
FIGURE 10.15 Three pathways that lead to cell death by apoptosis. In each case a signal comes from outside the cell.
This signal may be the binding of a ligand to its receptor (left panel); stress upon the cell caused by viral infection, DNA
damage caused by exposure to UV light, deregulation of the cell cycle, or other stresses (center panel); or from a cytotoxic
T cell (right panel). The signal leads to the activation of a procaspase by proteolytic cleavage. The activated caspase
begins a cascade that leads to apoptosis. Data for this figure came from Ashkenazi and Dixit (1998) and Raff (1998).
death receptors on the cell surface, which initiate apoptotic
anti-apoptotic protein is called Bcl-2, which blocks the action
pathways when their ligands are present and bind to them.
of Bax and interferes with the caspase cascade, among other
One such receptor is the Fas receptor (also known as CD95
activities. Bcl-2 is made by mature neurons, for example,
or Apo-1), which can be stimulated by Fas ligand present
in response to signals received when they form connections
on the surface of CTLs. Fas contains domains known as
with the target cells that they enervate. Immature neurons
death domains in the cytoplasmic region, and trimerization
do not make adequate amounts of Bcl-2 and die when nerve
of Fas induced by ligand binding results in the recruitment of
growth factor is removed. There is a complicated interplay
other cellular proteins by these death domains. This in turn
between Bcl-2 and related proteins and between Bax and
results in the activation, by cleavage, of a protease known as
related proteins that we are only beginning to understand.
caspase-8. A popular model for activation is that two mol-
Mitochondria are also important players in apoptosis.
ecules of caspase-8 must be brought into proximity so that
A decrease in the potential across the mitochondrial mem-
they cleave one another. Caspase-8 then cleaves other cas-
brane makes the cell more sensitive to apoptosis. Decreased
pases, thereby activating them in turn. At least 10 caspases
potential is accompanied by synthesis of reactive oxygen
are known, and they are normally present as inactive pro-
species and the release of cytochrome C into the cytoplasm,
caspases. A cascade of caspase cleavages leads, ultimately,
both of which are proapoptotic. Cytochrome C forms com-
to the activation of effector caspases. The effector caspases
plexes with other cellular proteins that promote the caspase
cause cell death by cleaving many cellular proteins, includ-
cascade. Bax, a proapoptotic protein, may exert its effect by
ing, for example, lamin in the nuclear membrane and a repair
lowering the mitochondrial membrane potential, and Bcl-2
enzyme called poly(ADP-ribose) polymerase.
may prevent this.
Another death receptor that may be present at the cell sur-
The importance of apoptosis for the regulation of the
face is a receptor for TNF (tumor necrosis factor). TNF can
organism is clear. Individuals with deficits in apoptotic
be secreted by CTLs, as well as by other lymphocytes, in
pathways may suffer from many diseases, including devel-
response to inflammatory signals. TNF receptor oligomeri-
opmental abnormalities, neurodegenerative disorders, or
zation also results in cleavage of caspase-8 and the induction
autoimmune diseases. In normal individuals, tumor cells
of the caspase cascade. The sensitivity of cells to the induc-
can only thrive if they avoid apoptosis despite the fact that
tion of apoptosis by these pathways thus depends on the con-
their cell cycle is deregulated, and mutations that suppress
centration of Fas and TNF receptor in the surface, and this
apoptosis are important for the development of tumors.
concentration can be regulated by other events.
Fas and TNF receptors are ubiquitous, present on most
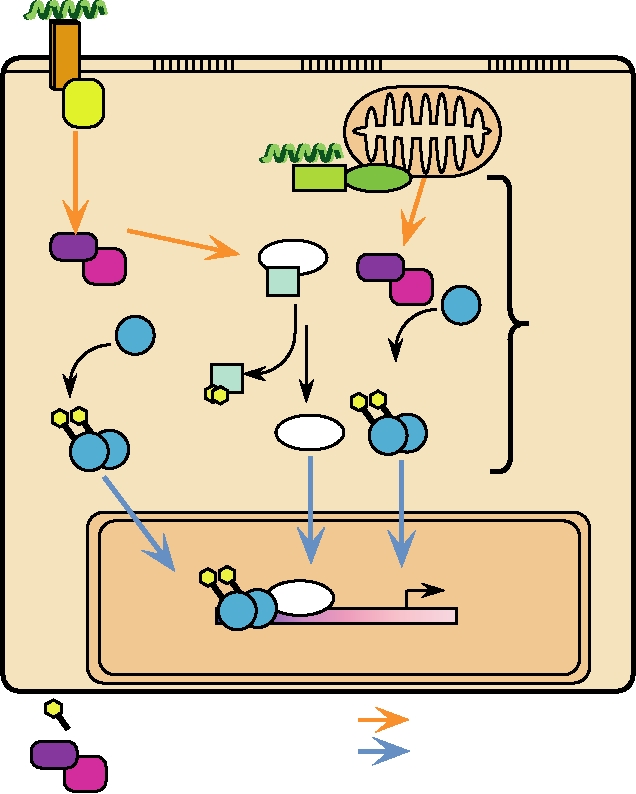
Interferons and Other Cytokines
cells. Many other receptors that can lead to apoptosis are
also known. Some of these are also ubiquitous, others are
The cytokines constitute a large family of proteins, defined
present on only a limited set of cells.
by their structure, that have important regulatory roles in an
The caspase cascade can also be initiated by other mecha-
animal. More than 30 cytokines have been described, and for
nisms. Withdrawal of growth factors, such as occurs during
some of these there is more than one gene. Most have a molecu-
development or occurs when activated T cells are no longer
lar mass of about 30 kDa. Many, but not all, are glycoproteins.
stimulated by interaction with their antigens, can lead to acti-
Some function as monomers but others act as homodimers or
vation of caspases. Events that damage DNA or the protein
homotrimers and at least one acts as a heterodimer. Cytokines
synthetic machinery can lead to apoptosis. At least some of
are inducible agents and constitutive production is normally
these apoptotic signals are delivered through changes in p53
low or absent. On induction, their production is short lived.
concentrations. p53 is a key player in the regulation of the
Cytokines effect their action by binding to receptors on the sur-
cell cycle (Chapter 7), and the apoptotic pathway is induced
face of target cells. These receptors bind cytokines with high
affinity--the dissociation constants range from 10-9 to 10-12
if its concentration is not tightly regulated. These mecha-
nisms involve Bax and related molecules, as well as Bcl-2
M. Binding to the receptor induces a cascade of events in the
and related molecules, as key players in the activation of the
target cell that leads to changes in gene expression within the
caspase cascade.
cell. These changes in gene expression may lead to many dif-
In addition to being able to initiate the caspase cascade
ferent effects. They are important in orchestrating the immune
indirectly by signaling through the Fas receptor or the
response by inducing cell proliferation in cells like T cells
TNF receptor, T cells can also initiate the caspase cascade
and B cells and causing changes in the state of differentiation
directly. They do this by introducing granzymes into the cell
of cells like dendritic cells. Hematopoietic cells are impor-
by way of channels formed by perforin secreted by the T
tant targets of all cytokines, although most cytokines have a
cell. Granzyme B cleaves procaspases downstream of cas-
diverse range of actions. The cytokine system is redundant in
pase-8 to begin the cascade.
that many cytokines evoke a similar spectrum of action, and it
Control of apoptosis is complex. Many cells make pro-
is pleiotrophic in that one cytokine may have many different
teins that render them less susceptible to apoptosis. One such
target cells. This system is also complex because many dif-
ferent cytokines are usually induced at the same time and
hematopoietic system (e.g., maintenance of lymphoid
they may act synergistically or antagonistically to achieve a
organs), whereas the proinflammatory chemokines recruit
result. Cytokines are important players in both the adaptive
immune cells to sites of infection, inflammation, or tissue
and innate immune systems. The importance of cytokines in
damage. The receptors for chemokines are distinct from
the maturation of T cells and B cells has been described. Fig.
those of cytokines. An example of a cytokine receptor is
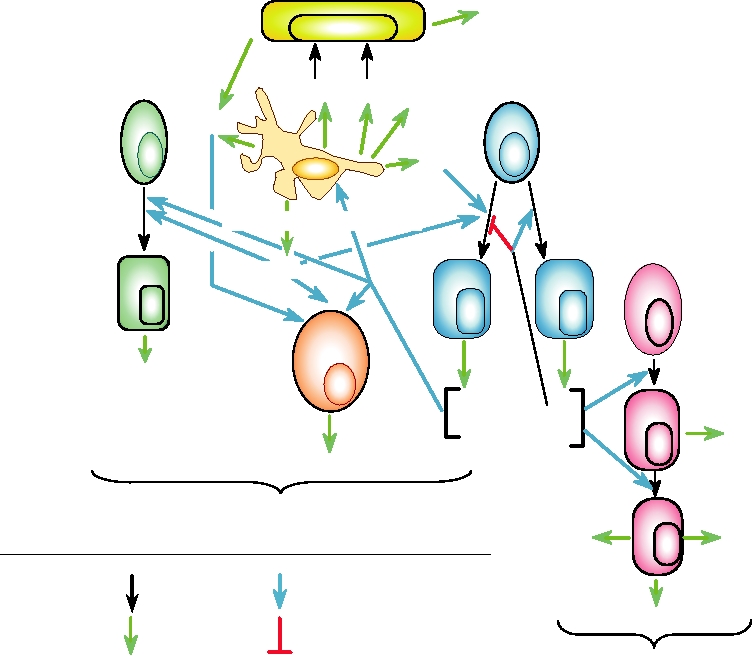
10.16 presents an overview of cytokine networks that illus-
described later in this chapter for interferon. Chemokine
trates their complexity, and a partial listing of cytokines is
receptors belong to the family of seven-transmembrane-
given in Table 10.5. Because of the complexity of their activi-
domain, G-protein-coupled receptors, and one was illus-
ties, we are only beginning to understand their many func-
trated schematically in Fig. 1.4B as the receptor for HIV.
tions, but as these functions begin to be understood, attempts
In some treatments, chemokines are considered a class of
are being made to use different cytokines therapeutically as
cytokines. Here, because these two classes of molecules dif-
indicated in the table.
fer in structure and mechanism of action, the term cytokine
A second family of proteins that play an important role
refers only to nonchemokine cytokines.
in the regulation of the immune response is the chemokines.
Chemokines are small proteins, 7080 amino acids in size.
Importance of Interferons in the Defense
More than 30 are known in humans. Some serve house-
against Viruses
keeping functions and are produced constitutively; others
are proinflammatory and usually inducible. Chemokines
Among the best known cytokines that function in the
serve to attract leukocytes. The housekeeping chemokines
defense against viruses are the interferons (IFNs). There
are important for the development and homeostasis of the
are two kinds of IFN, called type I and type II. The IFNs
Endothelial cell
MCP-1
IL-1b TNF-a
IL-6
CD8+T cell
CD4+T cell
IFN-a /b
IL-15
IL-18
Macrophage dendritic cell
Th1
Th2
B-cell
IL-12
Cytotoxic
T Lymphocyte
(CTL)
Natural
Killer
IFN-g
cell
LT-a
(NK)
IFN-g
IL-4
IL-5
IL-2
IL-10
LT-a
IgM
IL-13
IFN-g
Cell-mediated response to viral infection
Plasma Cell
IgG
IgA
Developmental
Activation
pathway
IgE
Secretion
Inhibition
Humoral response to viral infection
FIGURE 10.16 Overview of the cytokine networks important for innate and acquired antiviral immune responses.
IFN, interferon; IL, interleukin; Ig, immunoglobulin; TNF, tumor necrosis factor; LT, lymphotoxin; MCP, monocyte
chemotactic protein. Adapted from Griffin (1999) Figure 1 on p. 340.
TABLE 10.5
Some Cytokines and Their Therapeutic Uses
Functional
Name
Therapeutic
Side effects
group
(abbreviation)
Normal biological function
targets
of therapy
Antiviral
Type I interferon
Inhibits viral replication
Chronic hepatitis B,
Fever, malaise, fatigue,
(IFN-α,β)
Cytokines
hepatitis C, herpes
muscle pain; toxic to
zoster, papilloma
kidney, liver, heart,
viruses, rhinovirus,
bone marrow
HIV(?), warts
Type II interferon
Inhibits viral replication,
Lepromatous leprosy,
As above for Type I
(IFN-γ)
upregulates expression of class
leishmaniasis,
interferons
I and class II MHC, enhances
toxoplasmosis
activity of macrophages
Inflammatory
Tumor necrosis factor
Cytotoxic for tumor cells,
Anti-TNF in septic
Shock with marked
Cytokines
(TNF)
induces cytokine secretion
shock
hypotension
by inflammatory cells
Interleukin 1 (IL-1)
Costimulates T-helper cells,
Receptor antagonist in
???
promotes maturation of B cells,
septic shock
enhances activity of NK cells,
attracts macrophages and neutrophils
Interleukin 6 (IL-6)
Promotes differentiation of B cells,
stimulates Ab secretion by plasma cells
Regulators of
Interleukin 2(IL-2)
Induces proliferation of T cells, B cells,
Leprosy, local treatment
Vascular leak syndrome,
lymphocyte
and CTLs, stimulates NK cells
of skin lesions
hypotension, edema,
functions
ascites, renal failure,
hepatic failure, mental
changes, and coma
Interleukin 4 (IL-4)
Stimulates activity of B cells, and
proliferation of activated B cells,
induces class switch to IgG and IgE
Interleukin 5 (IL-5)
Stimulates activity of B cells, and
proliferation of activated B cells,
induces class switch to IgA
Interleukin 7 (IL-7)
Induces differentiation of stem cells,
increases IL-2 in resting cells
Interleukin 9 (IL-9)
Mitogenic activity
Interleukin 10 (IL-10)
Suppresses cytokines in macrophages
Septic shock
Interleukin 12 (IL-12)
Induces differentiation of T cells into CTLs
Interleukin 13 (IL-13)
Regulates inflammatory response in macrophages
Transforming growth
Chemotactically attracts macrophages, limits
Septic shock
Symptoms similar to
factor (TGF-β)
inflammatory response, promotes wound
those for IL-2,
healing
especially shock
and hypotension
Source: Data for this table came from Mims et al. (1993) p. 37.3, and Figure 7.13, and Kuby (1997) pp. 318, 319.
induce cells to become resistant to viral infection, an innate
extremely sensitive to infection by viruses. Viruses grow to
defense against viruses, but also play important roles in the
much higher titer in such animals and, in the more dramatic
adaptive immune response. The importance of IFNs in the
examples, virus infection may be lethal although infection
defense of mammals against viral infection has been shown
by the same virus in animals able to mount a normal IFN
by experiments in which an IFN response is ablated. Early
response may be asymptomatic. Many viruses have evolved
experiments in mice used injection of antibodies against
mechanisms to ablate the activity of IFNs, and in many cases
IFN to block its activity. More stringent ablation of IFN
it has been shown that viral mutants that have lost the ability
activity has been accomplished by using transgenic mice in
to resist IFN are severely crippled, again demonstrating their
which the receptor for either type I or type II IFN has been
importance in controlling viral infection. Interestingly, mice
abolished. In general, mice that lack a type I IFN response are
lacking a type I IFN response are able to handle bacterial
infections reasonably well. Conversely, mice lacking a type
Induction of IFN
II IFN response are extremely sensitive to bacterial infec-
Type I IFNs are induced upon the activation of a
tion but handle viral infections well. One known exception
number of PRRs, as described earlier. Induction may be
is vaccinia virus, which is lethal in mice lacking either IFN
direct (e.g., upon activation of TLR3, 4, 7, 8, 9 or RIG-1)
response. Although the relative importance of the two IFNs
or the result of production of other cytokines (e.g., TNF-α).
in the defense against viruses versus bacteria may differ,
The best studied activating agent that results in type
both are important in the defense against viruses.
I IFN production, and perhaps the most important agent,
is double-stranded RNA (dsRNA), whether infection is
by a DNA- or RNA-containing virus. Two dsRNA sen-
Types of IFNs
sors are known whose activation results in production of
Several characteristics of type I and type II IFNs are shown in
type I IFN, illustrated schematically in Fig. 10.17. One
Table 10.6. The type I IFNs are 165172 amino acids in length
sensor is TLR3 and the second sensor is a protein called
and have been classified into four different subfamilies, called
RIG-1. TLR3 is present in intracellular compartments
α, β, ω, and τ, of which α and β are the best studied. In humans
in many cells but present at the cell surface of fibrob-
there are 14 IFN-α genes and 1 IFN-β gene, none of which
lasts, where it senses external dsRNA. As noted earlier,
contain introns. IFN-β shares 2530% amino acid sequence
it is possible that intracellular TLR3 also senses exter-
identity with any particular IFN-α. In humans, IFN-β is glyco-
nal dsRNA that has been endocytosed or it may sense
sylated whereas IFN-α is not, but in mice both are glycosylated.
replicating RNA that enters the endosomal compart-
Thus, glycosylation is not a fundamental property distinguish-
ment. In any event, upon binding dsRNA, TLR3 sig-
ing α from β IFNs. IFN-α and IFN-β use the same receptors
nals through a protein called TRIF. In contrast, RIG-1,
and therefore evoke the same responses in target cells.
which has an RNA helicase domain that binds dsRNA,
Type II IFN contains only one member, IFN-γ. In humans
is present in the cytosol and therefore detects cytoplas-
there is one IFN-γ gene, which contains three introns. The
mic dsRNA. Upon binding dsRNA, RIG-1 interacts with
receptor used by IFN-γ is distinct from that used by type I
a protein called MAVS (it also has other names),which is
IFNs, but the phosphorylation cascade induced by IFN-γ con-
attached to the mitochondrial membrane by means of a
tains some elements that are shared with the type I cascade.
C-terminal transmembrane anchor. In either case, signal-
Thus the responses to the two IFNs are partially overlapping.
ing proceeds through a number of intermediate steps with
TABLE 10.6
Characteristics of the Interferons
Type I
Type II
INF-α
INF-β
IFN-γ
Characteristics
Alternative name
Leucocyte IFN
Fibroblast IFN
Immune IFN
Location
All cells
All cells
T-lymphocytes
Inducing agent
Viral infection or dsRNA
Viral infection or dsRNA
Antigen or mitogen
Number of species
14 (man),
1
1
(number of genes)
22 (mouse)
Chromosomal location of gene
9 (man),
9 (man),
12 (man),
4 (mouse)
4 (mouse)
10 (mouse)
Number of introns
None
None
Three
Size of IFN protein
165166 aa
166 aa
146 aa, dimerizes
Receptor for both IFN-α and INF-β consists of 2 polypeptides: IFN-α
Receptors
Receptor consists of two proteins: IFN-
R1 and INF-α R2, encoded on chromosome 21 (man) or 16 (mouse)
γR1 encoded on chromosome 6 (man)
or 10 (mouse) and IFN-γR2 encoded on
chromosome 21 (man) or 16 (mouse)
General functions
Anti-viral activity
Anti-viral activity
Macrophage activation
↑ MHC class Ia
↑ MHC class I
↑ MHC class I
↑ MHC class II on macrophages
NK cell activation, some antiviral activity
↓ MHC class II on B cells
↓ IgE, IgG production by B cells
↓ = downregulate; ↑ = upregulate.
a
Source: Data for this table came from Mims et al. (1993) Figure 12.9 and Fields et al. (1996) Table 3 on p. 378.
ds RNA
TLR3
TRIF
ds RNA
RIG-1 MAVS
TBK1
NFk B
IKKε
TBK1
IkB
Normal
IKKe
Interferon b
IRF3
Phosphorylation
Induction
Homodimerization
Phosphorylation
IRF3
Pathway
Degradation
Phosphorylation
IkB
Homodimerization
NF-kB
IRF3
IRF3
IFN-b
NF-kB
IRF3
NUCLEUS
Kinase activation
Phosphorylation
Translocation to nucleus
TBK1
Kinases
IKKe
FIGURE 10.17 The induction of interferon β. External double-stranded RNA signals through Toll-like receptor 3
(TLR3) to TRIF, and internal dsRNA signals to MAVS through the retinoic-acid inducible gene I (RIG-I) protein. MAVS
is the mitochondrial antiviral signaling protein. MAVS and TRIF both can activate the kinases TBK1 and IKKε, leading
to the phosphorylation and homodimerization of IRF3, and the phosphorylation and degradation of IκB, and enabling the
translocation of IRF3 and NFκB to the nucleus. In the nucleus activated IRF3 cooperates with NFκB to induce IFN-β
transcription. Adapted from Figure 1 in Freundt and Lenardo (2005).
the final result that transcription factors NFκB and IRF3
cell by any virus. However, some viruses are much more
are activated (Fig. 10.17). These then translocate to the
efficient at the induction of IFN than others, and it has been
nucleus and initiate transcription of a type I IFN gene.
postulated that viruses may differ in the extent of production
Transcription of type I IFNs is regulated at both the tran-
of dsRNA, or perhaps in the production of products that acti-
scriptional and posttranscriptional levels. Regulation at the
vate TLRs other than TLR3.
In contrast to type I IFNs, production of IFN-γ is largely
transcriptional level is complex and incompletely under-
stood. At the posttranscriptional level, IFN mRNAs contain
restricted to T cells and NK cells. Production is induced by
destabilization sequences in the 3′ nontranslated region and
agents that promote T-cell activation. These include exposure
have short half-lives. Thus, shutoff of IFN synthesis occurs
to antigens to which the organism has been presensitized or
fairly quickly once the gene is no longer transcribed.
exposure to IL-12, a cytokine produced by monocytes and
Almost all cell types produce IFN-α or -β, and thus
macrophages after infection by bacteria or protozoa. Other acti-
vators of T cells are also known that induce synthesis of IFN-γ.
induction of these cytokines follows infection of almost any
Many other cytokines are also induced following infec-
membrane protein that contains a cytoplasmic domain. The
tion by viruses. These include TNF-α, TNF-β, IL-1, IL-2,
type I IFN receptor is present in low abundance (around 103
IL-4, IL-5, IL-6, IL-8, and GM-CSF (granulocyte-macro-
receptors per cell) on all major types of cells. It has a very
high affinity for IFN (dissociation constant about 10-10 M).
phage colony-stimulating factor). The spectrum of cytokines
induced is different for different viruses. Some of the mech-
Upon binding of type I or type II IFNs to their cognate
anisms by which many viruses interfere with IFN or other
receptors, tyrosine kinases associated with the cytoplasmic
cytokines are described later.
domains are activated. In the case of type I IFNs, the kinases
activated are called TYK2 and JAK1. Upon activation, they
phosphorylate transcription factors STAT1 and STAT2 which
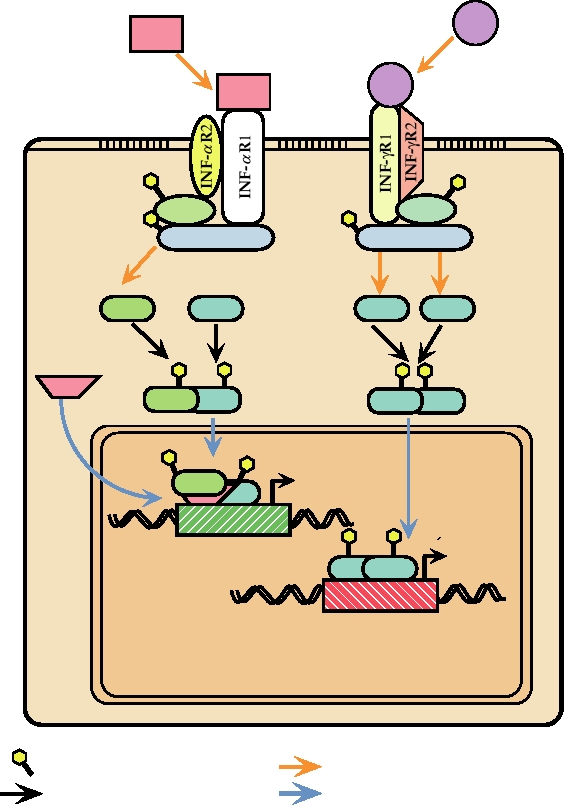
Interferon Receptors and Signal Transduction
then heterodimerize, recruit IRF-9 (p48), and translocate to
IFNs effect their responses by binding to specific receptors
the nucleus, where they stimulate the transcription of a large
on the cell surface. These receptors are composed of more
number of genes (Fig. 10.18). Most genes that are induced by
than one polypeptide chain, at least one of which is an integral
type I IFN have an upstream element referred to as ISRE, the
INF-g
INF-a
INF-g
INF-a
JAK-2
TYK-2
JAK1
JAK1
Recruitment
STAT1
STAT1
STAT1
STAT2
Phosphorylation
Dimerization
p48
ISRE
GAS
NUCLEUS
Interaction leading to phosphorylation
Phosphorylation
Protein-protein interactions
Migration to nucleus
FIGURE 10.18 Overlapping signal transduction pathways used for gene induction by IFN-α and IFN-γ, which bind
to different cellular receptors. Binding to their respective receptors leads to tyrosine phosphorylation of JAK1 and either
TYK2 or JAK2. These in turn phosphorylate STAT1 and STAT 2 proteins. The phosphorylated STAT proteins dimerize,
migrate to the nucleus, where they form complexes and bind to ISRE (interferon stimulated response element) or GAS
(IFN-gamma activation site), which are present upstream of interferon inducible genes, resulting in transcription of these
genes. Drawn from data in Fields et al. (1996) p. 379, Kalvakolanu (1999), and Nathanson et al. (1996) p. 123.
interferon stimulated response element. Induction of genes
effect is to stimulate the adaptive immune response. Both
controlled by this element is very rapid and happens within
type I and type II IFNs induce increased production of class
minutes after treatment with IFN. Activation is transient, as
I MHC molecules, thus leading to enhanced surveillance
one or more of the proteins induced act in a feedback loop to
by CTLs. Type II IFN also leads to increased production of
repress the continued transcription of these genes. In all, liter-
class II MHC in macrophages, which are important players
ally hundreds of genes are affected by IFN. The production
in the humoral response. The MHC response is augmented
of many genes is stimulated by IFN induction, but, in con-
by the induction of genes in the MHC cluster that encode
trast, many genes are instead repressed upon IFN induction.
components of the proteasome. These cause the proteasome
A few of the genes induced are listed in Table 10.7.
to become more active in producing peptides suitable for
In the case of type II IFN, the tyrosine kinases acti-
presentation by MHC molecules. Production of the TAP
vated are JAK1 and JAK 2. STAT1 is phosphorylated,
transporter system is upregulated, so that increased quanti-
homodimerizes, and translocates to the nucleus. The genes
ties of peptides are transferred across the ER membrane for
that are induced by IFN-γ are under the control of several
binding by MHC molecules. Either type of IFN leads to the
different regulatory elements, one of which is referred to as
activation of monocytes and macrophages, the activation of
GAS, the IFN-gamma activation site. Induction by IFN-γ is
natural killer cells, the activation of CTLs, and the modula-
slower and gene transcription continues for a longer period
tion of the synthesis of Ig by B cells, all important for the
immune response. IFN-γ also inhibits the growth of nonviral
of time after induction. The complex of genes induced by
type I and type II IFNs are overlapping because the tyrosine
intracellular pathogens and induces the increased expression
kinases associated with the type I and type II receptors and
of Fc receptors in monocytes. Fc receptors bind to a con-
the transcription factors that are phosphorylated are overlap-
served domain present in the heavy chain of all antibodies.
ping (Fig. 10.18). In addition, many genes contain cis-acting
This conserved domain is not involved in antibody binding
regulatory elements that respond to both type I and type II
but serves to interact with cells expressing Fc receptors and
IFN-induced transcriptional activators. A partial list of genes
to bind the C1q component of complement. Thus, antibodies
induced by IFN-γ is also given in Table 10.7.
bound to an infected cell or to a pathogen can recruit cells
such as monocytes or recruit complement to kill the patho-
gen or virus infected cell.
Biological Effects of Interferons
Both type I and type II IFNs are also key players in the
Type I or type II IFN induces the expression of many
innate immune defense against viruses. They are pyrogenic,
different genes that have important biological effects. One
inducing fever. High temperatures inhibit the replication of
TABLE 10.7 Genes Induced by Interferons
Induced bya
IFN-α
IFN-β
IFN-γ
Protein
Inducible element
Functions/phenotype
(2′-5′) (An) synthetaseb
(2′-5′) (An) synthesis/induction of antiviral state,
+++
+++
+
ISRE
esp. antipicornavirus
p68 Kinase (PKR)
+++
+++
+
ISRE
Protein kinase/induction of antiviral state
Indoleamine 2,3-dioxygenase
+
+
+++
Tryptophan degradation
γ56
+
+
+++
Trp-tRNA synthetase
GBP/g57
+
+
+++
Guanylate binding
MxA
+++
+++
+
Inhibits replication of influenza and VSV
IRF1/ISGF2
++
++
++
Transcription factor
IRF2
++
++
Transcription factor
MHC class I
+++
+++
+++
Upregulation of antigen presentation
MHC class II
++
Not ISRE nor GAS
Upregulation of antigen presentation
RING 12
+++
Proteosome subunit
RING 4
+++
+++
Putative TAP
β2 microglobulin
+++
+++
+++
MHC light chain
a
The strength of the induction is indicated by the number of plus signs.
b
Full induction also requires dsRNA.
Source: Adapted from Nathanson et al. (1996) p. 124.
many viruses or other pathogens. It has been proposed that
require that dsRNA be present for them to be active. Thus,
the major symptoms of influenza infection, which include
not only is dsRNA a primary inducer of IFN synthesis, but
high fever as well as muscle aches and pains, are due to the
these two pathways induced by IFN are also dependent on
induction of IFN rather than to virus replication per se. Both
dsRNA for their activation. This dependence on dsRNA sug-
IFNs also induce what has been called the antiviral state,
gests that it is a common product in viral infection, whether
described next, in which cells are less susceptible to or
the virus is DNA or RNA, but is not normally present in
resistant to infection by viruses.
uninfected cells.
One inhibition pathway involves 2′-5′ oligo(A) syn-
Thus, the induction of IFNs is important for the control of
thetases, 2′-5′ OS. These synthetases, of which several are
viral infection at more than one level. Induction of the anti-
viral state results in lower yield of virus and the stimulation
known, some of which have different subcellular locations,
of immune responses leads to a more effective and faster
polymerize ATP into oligoadenylates that are joined in a
2′-5′ linkage. 2′-5′ Oligo(A) synthetase is induced by IFN but
clearance of the viral infection. Of interest is the fact that
requires dsRNA as a cofactor for activity. 2′-5′ Oligo(A), in
both types of IFNs inhibit cell growth, and treatment with
IFN or with inducers of IFN has been effective in the treat-
turn, is a cofactor for a latent ribonuclease, RNase L, which
ment of at least some types of cancer.
is present in all animal cells, but is inactive in the absence
of 2′-5′ oligo(A). Once activated by its cofactor, which is
bound tightly enough so that the two can be coimmunopre-
The Antiviral State
cipitated, RNase L can cleave single-strand mRNAs. 2′-5′
Expression of the genes induced by interferon results
Oligo(A) is hydrolyzed by a phosphodiesterase present in
in the establishment of the antiviral state in cells, in which
cells, and activation of RNase L is transient.
viruses fail to replicate or replicate to much lower titers.
Thus, RNase L results in a degradation of mRNAs only
The antiviral state is multifaceted and interference with the
in those cells in which dsRNA is present. The picornaviruses
replication of viruses may occur at different stages of their
are particularly sensitive to the action of RNase L, and it has
replication cycle. The effect, therefore, depends on both the
been well established that activation of RNase L is a primary
virus and the host cell. Furthermore, interference can occur
mechanism by which IFN interferes with the replication
at more than one stage of replication for some viruses, and
of these viruses. Other viruses seem to be less affected by
such viruses tend to be more sensitive to the activities of
RNase L, and the possible role of this enzyme in combating
interferon than others.
other virus infections remains to be determined.
Interference with the virus replication cycle may occur
The second pathway leading to the inhibition of trans-
very early, during penetration and uncoating of the virus.
lation of viral mRNAs involves a protein kinase known as
This occurs with SV40 and the retroviruses, for example.
PKR. PKR is present at low levels in most cells, but its con-
The proteins that are responsible for interference at these
centration increases on IFN induction. It requires dsRNA for
early stages of infection are unknown. For some viruses
activity. On binding dsRNA, PKR is autophosphorylated on
the transcription of the infecting viral genome is inhibited,
serine and threonine residues. Once activated, it phosphor-
examples being influenza, vesicular stomatitis virus, and
ylates the translation initiation factor, eIF-2. Phosphorylated
herpes simplex virus. A host protein known as Mx, which
eIF-2 cannot be recycled and protein synthesis is shut down.
is induced by interferon, is responsible, at least in part, for
The importance of PKR for inhibiting viral replication is
interfering with the transcription of influenza virus. In mice
shown by the fact that several viruses encode inhibitors of
that are unable to produce the Mx protein, as is the case for
its activity (see later). In particular, it is believed that reo-
most inbred strains of mice, infection with influenza pro-
viruses, adenoviruses, vaccinia virus, vesicular stomatitis
duces a fatal outcome, demonstrating the importance of this
virus, and influenza virus, among others, are sensitive to the
gene. At a later stage in the infection cycle, interferon treat-
effects of this enzyme.
ment results in reduced translation of many viral mRNAs,
PKR binds dsRNA and thus is another sensor for dsRNA
and the mechanisms by which this occurs are described next.
within the cytoplasm of the cell. Whether it has any activity
Finally, some interferon-induced products interfere with
upon activation by dsRNA other than shutdown of protein
virus assembly. This occurs with the retroviruses, vesicular
synthesis is not clear, but PKR is known to have important
stomatitis virus, and herpes simplex virus, but the proteins
roles in the regulation of cellular processes in addition to its
responsible are unknown.
antiviral activities. For example, it has a role in regulating
several signal transduction cascades. In association with other
factors, PKR can activate NFκB, which is proinflammatory.
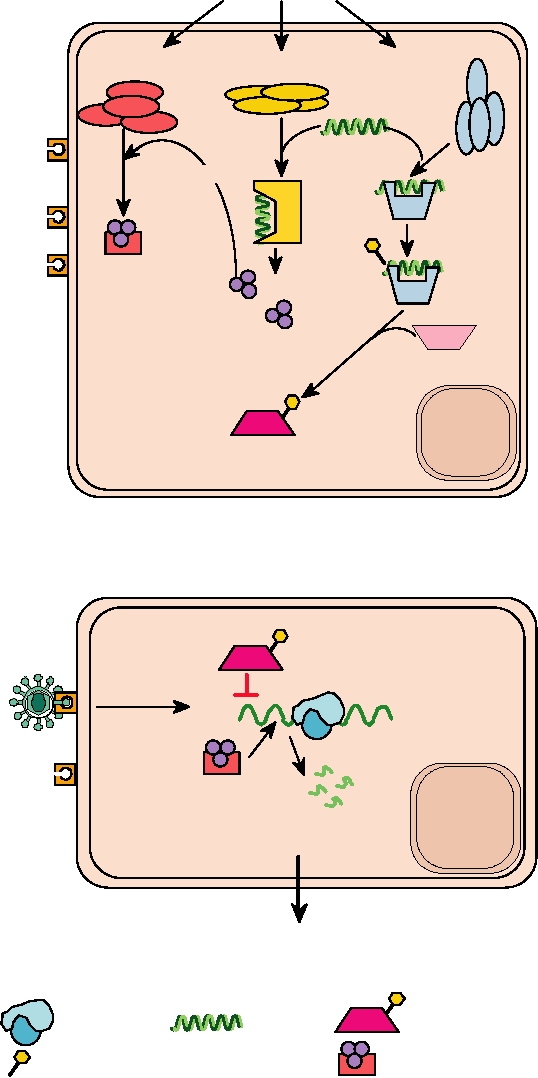
Interference with Translation of Viral mRNAs
It also has a role in the activation of certain MAP kinases and
Two distinct pathways induced by IFN result in interfer-
a stress-related protein kinase. It is involved in the regulation
ence with the translation of viral mRNA. These are illus-
of the cell cycle and if PKR is nonfunctional, unregulated cell
trated schematically in Fig. 10.19. Both of these pathways
proliferation and neoplastic transformation occur.
A. Development of the antiviral state
Induction by IFN
Latent RNase L
Latent 2'-5' OS
Latent PKR
dsRNA
ACTIVATION
ACTIVATION
PKR
2'-5' OS
AUTOPHOSPHORYLATION
RNase L
SYNTHESIS
2'-5'
oligo(A)
EIF2
PHOSPHORYLATION
EIF2
NUCLEUS
UNINFECTED HOST CELL
B. Virus attempts to replicate in a cell in the antiviral state
EIF2
TRANSLATION INITIATION BLOCKED
Uncoating
Viral
mRNA
RNaseL
VIRAL mRNA DEGRADED
NUCLEUS
NO VIRAL REPLICATION
Phosphorylated EIF2
EIF2
Ribosome
dsRNA
Phosphate group
Activated RNase L
FIGURE 10.19 The antiviral state. (A) Development of the antiviral state begins with the action of interferon on an
uninfected cell. The result of the signal transduction cascade shown in Figure 10.18 is the induction of expression of up to
100 genes, of which three are shown: RNase L, the 2′-5′ oligo(A) synthetase (2′-5′ OS), and the dsRNA-dependent protein
kinase (PKR). These proteins are latent until they are activated by viral infection. PKR and 2′-5′ oligo(A) synthetase
are activated by dsRNA that is produced during viral infection. Once activated, PKR autophosphorylates itself, and
then phosphorylates EIF2. The activated synthetase makes trimeric oligonucleotides which in turn activate RNase L.
(B) Phosphorylated EIF2 and activated RNase L are characteristic of the "antiviral state" in which a eukaryotic cell
is refractory to infection by a wide variety of viruses. Phosphorylated EIF2 cannot serve to initiate translation of mRNA
by ribosomes and activated RNase L degrades mRNAs, both viral and cellular, so protein synthesis stops. Without protein
synthesis no virus replication can take place, but the inhibition of protein synthesis is transient and the cell may recover.
Adapted from Nathanson et al. (1996) Figure 6.8 on p. 125.
Degradation of mRNA and shutoff of protein synthesis
investigators and by biotech companies to use these mecha-
are drastic events that represent a defense of last resort. The
nisms to specifically turn off viral genes as a method to con-
dependence of both pathways on dsRNA means that only
trol viruses have followed. It is now clear that almost every
cells that contain dsRNA, presumably only virus-infected
cell can produce such interfering RNAs. These RNAs are
cells, will shut down as a result of these pathways. To sim-
used in the determination of the fate of the cell, in protecting
plify somewhat, one effect of IFN is to prepare the cell for
organisms from transposons and genomic rearrangements,
instant shutdown if it becomes infected, while leaving unin-
in physiological regulation, and in brain morphogenesis.
fected cells essentially alone.
The fact that several viruses have been found to encode
products that interfere with gene silencing (described later
in this chapter) indicates that the gene-silencing pathway is
Therapeutic Uses of Interferon
also important for the control of at least some viruses.
Since the discovery of IFN some 50 years ago, there has
There are two sources of these small RNAs used for gene
been great hope and expectation that IFN therapy would be
silencing. One source is long dsRNA, whether supplied
useful for the treatment of viral infections. In early stud-
externally or synthesized within the cell, which gives rise to
ies, IFN was induced in patients by injection of dsRNA,
RNAs called siRNAs (for short interfering RNAs). The long
but quantities of recombinant IFN-α suitable for injection
dsRNAs can be shortened by RNaseIII but are ultimately
are now available. In general, IFN-α therapy has been dis-
cleaved by the ribonuclease DICER into short dsRNAs that
appointing for treatment of viral infections in humans,
are typically 21 nucleotides long with 2 nucleotide over-
hangs at each 3′ end. These short molecules are then deliv-
although it is useful in a number of diseases (Table 10.5).
Infection with hepatitis B or hepatitis C viruses often results
ered to the RISC (RNA-induced silencing complexes) where
in chronic infection. In at least some of these chronically
they interact with one or more argonaute proteins (Ago1,
infected patients, the virus load can be decreased, or the dis-
Ago2). In the RISC complex the "sense" strand is selectively
ease may go into remission, upon long-term treatment with
removed, and the resulting "guide strand" ssRNA binds to
recombinant IFN-α. However, side effects of IFN treatment
the complementary portion of mRNA from the gene to be
often limit the dose and duration of treatment that can be
silenced. Depending upon the gene, the organism, and the
used. IFN-α has also been useful in treatment of infection
degree of sequence identity between the siRNA and the tar-
with papilloma viruses. More recently, combination therapy
get sequence the silencing may be accomplished by cleavage
in which IFN-α has been combined with other drugs that
of the mRNA or by repression of translation of the mRNA.
interfere with virus replication, such as ribavirin, which
The siRNA may also be used to repress the transcription of
depletes GTP pools in cells, have often proved more suc-
a gene through chromosome modification, but this proc-
cessful than treatment with IFN-α alone, and such methods
ess is not understood at present. Figure 10.20 illustrates the
for treatment of chronic virus infection continue to evolve.
production and use of siRNA to silence genes.
During clinical trials with IFN-α, it was found that this IFN
The second source of interfering RNAs is ssRNA that
is useful for the treatment of at least some cancers, including
is transcribed from the genome of many (all?) organisms,
hairy-cell leukemia and AIDS-related Kaposi's sarcoma. It
including humans. These RNAs form long hairpins of imper-
is assumed that this control is based on the inhibition of cell
fect complementarity, which are processed by a nuclear
growth caused by IFNs. Clinical trials using IFN-α, as well
enzyme called Drosha to form pre-micro RNA (pre-miRNA)
as other cytokines, for treatment of other viral diseases and
of about 70 nts. These are exported to the cytoplasm by the
other neoplasias continue and it is to be expected that further
protein Exportin5 where they are cleaved by DICER into
uses for these agents will be found (Table 10.5).
dsRNAs about 21 nucleotides long, and delivered to RISC.
Here the sense strand is removed and the resulting miRNA
performs the same function as do siRNAs. miRNAs, how-
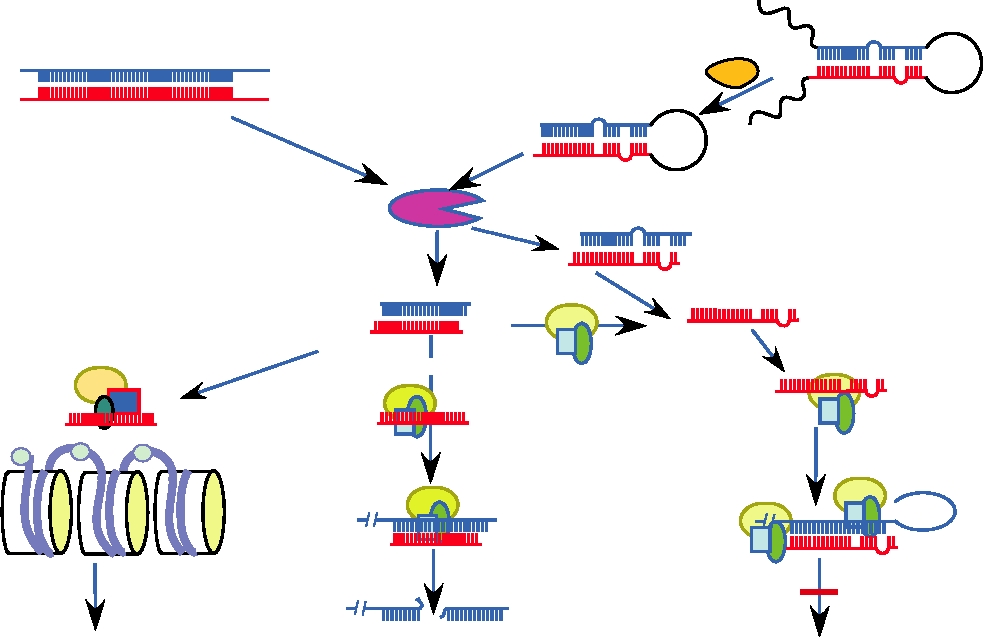
Gene Silencing
ever, often contain one or more mismatches with their tar-
gets, unlike siRNAs, and thus miRNAs are more likely to
It has been known for many years that plants use RNA
function as translational repressors rather than causing
molecules 21 to 25 nucleotides in length, sometimes referred
mRNA degradation. However, some miRNAs do cause
to as RNAi (i for interference), to silence genes. The discov-
mRNA degradation, and some siRNAs cause the inhibition
ery in 1999 that a similar mechanism exists in animals, in
of translation, so that the functions of these two RNAs are
this case in the worm Caenorhabditis elegans, and that this
overlapping, as are the pathways that lead to their formation
mechanism is used to control a developmental pathway in
(Fig. 10.20). There are approximately 400 miRNA genes in
the worm, created a great deal of excitement and resulted in a
humans, grouped by sequence into 350 families. Many of
Nobel Prize for Andrew Fire and Craig Mello (see Table 1.1).
these are encoded in the 3′ NTRs of mammalian genes and
Since 1999 a great deal of work has elucidated the details
a small fraction of them are tissue specific. Each miRNA
of the mechanisms involved and extended the findings to
can have many targets, since for most genes a 7-nucleotide
many other animals, including mammals. Attempts by many
Pre-miRNA
Long dsRNAs
Drosha
Pre-miRNA
RNaseIII
DICER
Cleavage into 21-nt dsRNAs
5P
with 3 overhangs
3
P5
3
siRNA
RISC
5P
ature miRNA
3 m
P5
P3
5
3
miRNP
Selective degradation of sense strand
ITS
Ago 1
A
P5
Ago 2
3
RISC
R
P5
P5
3
3
DNA methylation
Me
Me
Me
Ago 2
AAA
Histones
CAP
AAAA
CAP
P5
P5
3
3
Translational
CAP
AAAA
repression
Transcriptional
mRNA degradation
gene silencing
FIGURE 10.20
Mechanisms of RNA interference. Long dsRNAs are cleaved by RNaseIII and subsequently processed
by DICER into siRNAs, which are short dsRNAs of 2122 nts with 2-nt overhangs on the 3 termini. siRNAs then enter
the RNA-induced silencing complex (RISC) where the sense strand is selectively degraded. miRNAs are encoded as
incompletely self-complementary hairpins in viral and cellular genomes. They are cleaved by Drosha in the nucleus and
the pre-miRNA exported to the cytoplasm by Exportin5. There they are also processed by DICER. siRNAs silence genes
by binding in the context of RISC to mRNAs that are exactly complementary and causing mRNA degradation. miRNAs
often contain some mismatches and generally exert their effects by inhibiting translation. siRNAs can also enter RNA-
induced transcriptional silencing complexes (RITS) which recruit an enzyme to methylate DNA in chromatin, turning it
into inactive heterochromatin. Both RITS and RISC contain argonaute proteins (Agos). The figure is adapted from those
in Novina and Sharp (2004) and Schütz and Sarnow (2006).
match is sufficient for silencing. It is clear that these miRNAs
tions, although expression of the RNA by expression vec-
are important for the development of the organism and for cell
tors in the cell has been used in a few cases. These initial
regulation, but the many functions of these RNAs have as yet
experiments highlight some of the problems with adapt-
to be unraveled.
ing siRNA to therapeutic use. First, many cells do not take
There are ongoing attempts to develop gene silencing
up small RNAs easily or efficiently and expression vec-
as a new antiviral strategy, and numerous trials have been
tors may be required, which pose another set of problems.
made using a variety of viruses. Table 10.8 summarizes
Second, small RNAs have a short half-life in the average
results in both cell culture and in experimental animals
cell. Attempts to extend the half-life have included altering
the siRNAs in a number of ways, such as 2′-O methyla-
for a number of viruses. For many viruses, including HIV,
polio, and foot-and-mouth disease virus, treatment of cells
tion of the bases or covalent attachment of cholesterol at
the 3′ terminus. Third, it is unknown how specific siRNA
with siRNA either previous to or concommitantly with viral
infection reduced viral replication significantly. For the
or miRNAs would be for their intended targets and whether
experiments in cell culture, uptake of siRNA was usually
cellular mRNAs that might share some sequence identity
induced by treatment with lipofectamine or other polyca-
with the gene to be silenced might be downregulated in a
TABLE 10.8 Viruses Suppressed by siRNA
Cell type or
Reduction in viral
Virus
organism
Delivery protocol
Genes targeted
yield
Viral replication in cultured cells
HIV
Astroglioma cells
Lipofectamine
Polymerase, Nef
7080%
HIV
Human primary T cells
Lentiviral vector
CCR5
???
Cultured cell lines
Lipofectamine
Five viral genes and LTR
???
Poliovirus
HeLa Cells
Lipofectamine
Capsid, polymerase
9095%
HRSV
Cultured cell lines
Lipofectamine
???
???
5′NTR, 3′NTR, VPg, VP4, Pol
FMDV
BHK-21 cell lines
Lipofectamine
9099.9%
Flock house virus
Drosophila cell line
Endogenous cellular siRNA
???
Nearly 100%
Vaccinia
HeLa cells
???
E3L-specific siRNA
97%
Influenza
MDCK, CEF, chicken eggs
Plasmids
NP, PA
???
LCMV
Cultured cells
Recombinant adenovirus vector
L, Z1
Cures chronically infected
cells
Viral replication in whole animals
VSV
C. elegans
Gene products of rde-1, rde-4
???
???
Influenza
Mice (lung)
Inhale siRNA with polycations
???
99%
HRSV and
Mice (lung)
Inhale siRNA without
Essential viral genes
Protected from simultaneous
parainfluenza
transfection reagents
viral challenge
HSV-2
Mice (vagina)
Oligofectamine
UL27 and UL29
Protected from challenge up
to 3 hours later
Hepatitis B
Mice (intravenous)
siRNA in liposomes
???
???
Coxsackie B3
Mice (intravenous)
Hydrodynamic injection
???
Reduces virus by 6 logs
Japanese
Mice (intracranial)
Lipid complexed lentiviral
cd-loop domain of envelope
Protects against both JE
encephalitis
vector
protein
and WN encephalitis
Abbreviations: HIV, human immunodeficiency virus; LTR, long terminal repeat; HRSV, human respiratory syncytial virus; FMDV, foot and mouth disease
virus; LCMV, lymphocytic choriomeningitis virus; VSV, vesicular stomatitis virus; HSV-2, herpes simplex virus 2; JE, Japanese encephalitis virus;
WN, West Nile virus.
Source: Data from McManus and Sharp (2002), Lecellier et al. (2005), and Dykxhoorn et al. (2006).
"bystander effect." Obviously, such bystander downregula-
Preliminary experiments in mice have also been performed
tion could potentially be deleterious.
to explore ways of delivering siRNAs to appropriate tissues
More detailed experiments with HIV in cell culture
in sufficient quantities to effect viral silencing (Table 10.8).
have used not only siRNA exogeneously supplied but also
Some success has been achieved for viruses affecting the
lentiviral vectors expressing siRNAs to target a variety of
lungs, for lung tissue appears to be able to take up RNA even
HIV genes, the 5′ LTR, and even the HIV coreceptor CCR5
without transfection reagents such as lipofectamine. Similarly,
(referred to in Table 10.8). What has become apparent from
viruses affecting the genital tract can be suppressed by siRNA
these studies is that HIV mutates rapidly under pressure
inserted into the vagina with oligofectamine. Hepatitis B
from siRNA inhibition and resistant variants arise quickly.
infection has been treated with siRNA introduced intrave-
Even single nucleotide substitutions in the center of the core
nously in liposomes. Other intravenous approaches have so
recognition sequence can make the virus resistant to silenc-
far been less successful, although a Coxsackie B3 infection
ing. Thus, resistant variants can arise that have an unaltered
in mice could be suppressed by a million-fold after siRNA
amino acid sequence because they possess only silent muta-
was introduced by hydrodynamic injection. Hydrodynamic
tions. Similar results with picornaviruses have implied that
injection, however, carries significant risk of cardiac failure
any successful RNAi strategy must involve multiple siRNAs
and is not an option for human treatment. Thus at the current
directed at a variety of targets so as to reduce the probability
time, siRNA appears to be a molecule with much therapeutic
that resistant variants will quickly arise.
potential but no proven efficacy.
Search WWH :