Host Defenses against Viral Infection
and Viral Counter defenses
INTRODUCTION
Major Histocompatibility Complex
Both cellular immunity and humoral immunity require
On infection of a mammal by a microorganism, a complex
the activation of a class of lymphocytes called T cells
response is generated that attempts to eliminate the infec-
(T from thymus, where these cells mature). T cells recognize
tious agent. These responses can be conveniently grouped
peptide antigens 820 amino acids in length that are pre-
into two categories, innate immune responses and adap-
sented to them by cell surface proteins encoded in the major
tive immune responses. The adaptive or acquired immune
histocompatibility complex (MHC) locus (in humans, the
response requires time to develop, is specific to the invading
MHC is called HLA, from human lymphocyte antigen, and
pathogen, and is followed by immunologic memory that usu-
is encoded in chromosome 6). The two types of MHC mol-
ally renders the host immune, or at least less susceptible, to
ecules that present antigenic peptides are called class I and
subsequent infections by the same organism. The two most
class II. Both class I and class II MHC molecules are integral
important components of this response are the production
membrane proteins that are composed of two polypeptide
of cytotoxic T lymphocytes (CTLs) and the production of
chains.
humoral antibodies. Innate responses, in contrast, generate
early responses to infection, are not specific to the patho-
gen, and do not render the organism resistant to subsequent
Class I and Class II MHC
infection by the same pathogen. Cytokines such as inter-
The MHC class I molecule is a heterodimer composed of
feron are among the most effective innate responses. Innate
a heavy chain of about 350 amino acids, which is encoded
and adaptive immune responses do not function indepen-
within the MHC locus, and a light chain of about 100 amino
dently of one another: The proper functioning of the immune
acids, β2 microglobulin, which is encoded elsewhere. The
system requires the activities of both, and, in fact, the activa-
structure of an MHC class I molecule is shown schematically
tion of the adaptive response requires the prior activation of
in Fig. 10.2A, and as determined by X-ray crystallography in
the innate system. The various activities of the two systems
Fig. 10.2B. The MHC class I heavy chain consists of three
constitute a multifaceted, interactive, and complex series of
extracellular domains called α1, α2, and α3, a transmem-
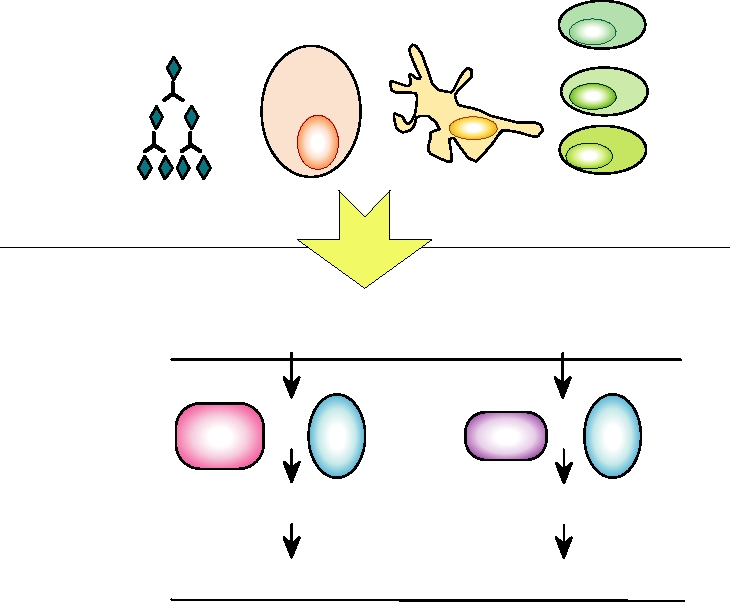
responses to infection by a pathogen. Figure 10.1 illustrates
brane domain, and a cytoplasmic domain. β2 microglobulin
some of the cells and other participants involved in innate
forms a fourth extracellular domain and is held in the com-
and acquired immunity.
plex by noncovalent interactions. The α1 and α2 domains,
which are structurally related to one another, form a platform
ADAPTIVE IMMUNE SYSTEM
with helical walls. The walls form a groove in which the
antigenic peptide, consisting usually of 810 amino acids, is
anchored. The α3 domain and β2 microglobulin are homolo-
The adaptive immune system contains two major arms.
The cellular arm leads to the production of CTLs, also called
gous (derived from a common ancestral polypeptide by gene
killer T cells. The humoral arm leads to the production of
duplication) and are members of the immunoglobulin (Ig)
antibodies that are secreted by B cells. T-helper cells are
superfamily. They share some sequence identity and have
important players in orchestrating both of these arms.
common structural features.
Innate Immunity
Complement
Immune System
Macrophages
Natural Killer
Monocytes
Cascade
Players
Cells
Eosinophils
Neutrophils
Cytokines
PRRs
Acquired Immunity
Pathogen/Antigen
Intracellular Antigens
Extracellular
Cells infected with
Antigens
Viruses, Rickettsia, or Mycoplasma
Bacteria, Viruses
Immune System
TH1
+
TH2
+
B Cell
CTL
Players
Immediate Effect
Cell killing by CTLs
Soluble Antigen, Activated B Cell
Result
Humoral Immunity
Cell-Mediated Immunity
Antigen Presentation
Class II MHC
Class I MHC
FIGURE 10.1 The mechanisms of innate and acquired immunity are integrated to provide the basis for humoral and
cellular immunity. PRRs are pattern recognition receptors. Adapted from Mims et al. (1993), p. 5.8.
The structure of MHC class II molecules is similar to
exceedingly polymorphic and there are hundreds of different
that of class I molecules, as illustrated in Fig. 10.2A. Class
alleles within the human population. The haploid number
II molecules are composed of a heterodimer of two proteins
of genes in humans that encode heavy chains used in class
encoded within the MHC locus. These two proteins, des-
I MHC molecules is three (called HLA-A, -B, -C), and a dip-
ignated α and β, each contain two extracellular domains
loid individual can make up to six different class I MHC
(and thus the assembled molecule contains four extracel-
molecules with differing requirements for anchor residues.
In the case of class II MHC, six α and seven β genes have
lular domains as does class I MHC). Both proteins are
anchored in the plasma membrane by membrane-spanning
been identified in humans (of which the most important
anchors and have cytoplasmic domains. The distal α1 and
loci are called DR, DQ, and DP). Thus, any individual can
β1 domains form a platform with a groove that binds an
present very many different peptides to T cells. Note that
antigenic peptide for presentation to T cells, but in this case
since the MHC is polymorphic, different individuals present
the peptide is longer, usually 1418 amino acids in length.
different peptides to T cells.
The proximal domains, α2 and β2, are members of the Ig
superfamily.
T-Cell Recognition of Peptide Antigens
The number of peptides that can be presented by an
individual class I or class II molecule is large. Only cer-
T cells express a T-cell receptor on their surface, which is
tain residues in the peptide, called anchor residues, inter-
able to recognize a specific peptide presented in the context
act specifically with the MHC molecule. The remainder of
of class I or class II MHC molecules. The T-cell receptor
is a heterodimer formed by one α and one β chain, or by
the peptide can vary in sequence. Furthermore, the MHC is
Class II MHC
Class I MHC
SS
SS
a2
a1
b1
a1
SS
S
2 S
S
S
a3
a2
b2
b 2m
S
S
S
S
Plasma
membrane
Cell cytoplasm
Peptide-binding
cleft
a 2 domain
a 1 domain
b 2 microglobulin
a 3 domain
FIGURE 10.2
Upper panels: schematic representations of MHC class I and MHC class II molecules.
The orientation of these molecules at the cell surface is indicated, as are the domain structures of the extracellular
portions of the proteins, the membrane-spanning domains and the cytoplasmic domains, and how they function in
the cell. The yellow and green spheres represent bound peptide antigens. Lower panel: three-dimensional ribbon
diagram of the structure of MHC-1 (HLA-A2), as determined by X-ray crystallography. From Bjorkman et al.
(1987) as reprinted in Kuby (1997).
one γ and one δ chain, as illustrated in Fig. 10.3. The great
domains. V and J, or V, D, and J, are first joined at the DNA
majority of circulating T cells possess receptors formed by
level by a process that deletes the intervening DNA. The
αβ dimers.
combined VJ or VDJ is then joined to C by splicing of the
During maturation of T cells, three or four separate regions
pre-mRNA transcript. Multiple copies of each of the four
of the genes for α or β, respectively, are brought together by
segments exist in the germ line and combinatorial joining
of these results in a very large number of possible α or β
deletion of the intervening sequences, with the rearrange-
ments to form the β chain occurring first. This process is
subunits. Furthermore, the joining of VJ and VDJ is impre-
illustrated in Fig. 10.4. The regions are the V (variable), D
cise, and additional diversification results from repair of
(diversity), J (joining), and C (constant) regions for β, or
the joining regions. Combinatorial joining of α and β subu-
the V, J, and C regions for α. The V and C regions belong to
nits results in the production of an even larger repertoire of
the Ig superfamily, whereas D and J are shorter, unrelated
T-cell receptors. An estimate of the possible diversity of
a or g chain
b or d chain
T-cell receptors, sometimes referred to as the theoretical
repertoire, is shown in Table 10.1. The theoretical repertoire,
NH2 NH2
how many T-cell receptors could possibly be formed, is
Variable
S
S
perhaps 1018, which is an exceedingly large number.
Regions
S
S
Once any individual T cell develops and expresses a
T-cell receptor, no further recombination occurs and the
receptor does not change thereafter. The function of T cells,
S
S
Constant
simply stated, is to discriminate self from nonself, and
Regions
S
S
T cells that express an appropriate receptor that serves
SS
this function are selected in the thymus (see later) and
Plasma
released into the circulation, where they are long lived.
TM
membrane
Any individual has many, many T cells in the body and
these continue to develop throughout life. The repertoire
Cell cytoplasm
COOH COOH
present in any human at any one time certainly exceeds
108 different T-cell receptors, expressed on different T
cells.
Each different T-cell receptor is potentially able to
recognize a different peptide epitope (the epitope is the
FIGURE 10.3
Structure of the T-cell receptor (TCR). Receptors are
surface of the peptide that interacts with the T-cell recep-
heterodimers of α/β or γ/δ TCR chains. Each chain has a transmembrane
tor). The T-cell receptor recognizes the peptide epitope in
anchor (TM). The C-terminal domains and the TM are relatively constant in
the context of class I or class II MHC; that is, the T-cell
sequence, but the N-terminal domains are variable. Adapted from Chen and
Alt (1997) Figures 7.6 and 7.7 on pp. 344 and 345.
V1
Vd n Dd1Dd 2 Jd1 JdD Cd
5
3
Ja1 Ja 2
2
Va n Vd 1
Ja n
Ca
a
Germ-line a-chain DNA
V-J Joining
3
5
Va1
Va 2
Va Ja Ja n
Ca
Rearranged a-chain DNA
Transcription
mRNA splicing
a chain
Translation
Va
Ja Ca
Protein product heterodimer
Vb
Jb
Db
Cb
b chain
Transcription
mRNA splicing
Translation
Db
Jb
5
Cb 2
Cb1
Db 2
Vb 14 3
Vb1
Jb
Vb
Rearranged β-chain DNA
V-DJ joining
-J joining
D
Jb
Jb
5
Vb14 3
Cb1
Cb2
Vb1
Vb n
Db
b2
Germ-line
b -chain DNA
FIGURE 10.4 Gene rearrangements to generate T-cell receptor diversity. The example shown generates an αβ T-cell
receptor. The α chain undergoes a VαJα joining, whereas the β chain undergoes two joins: Dβ to Jβ followed by Vβ to
DβJβ. Primary RNA transcripts are spliced to give mRNAs in which RNA sequences encoding VJ or VDJ are joined to
RNA sequences encoding constant domains, Cα or Cβ. These spliced mRNAs are then translated into the α and β chains
of the TCR. Adapted from Kuby (1997) Figure 11.6 on p. 269.
TABLE 10.1
Comparison of Diversity in Human Immunoglobulin and T-Cell Receptor Genes
Immunogobulins
αβ T-cell receptors
γ δ T-cell receptors
Light chains
κ chain
λ chain
α chain
β chain
γ chain
δ chain
Mechanism of diversity
Heavy chains
Multiple germ-line gene segments
V
65
40
30
~70
52
12
>4
D
27
0
0
0
2
0
3
J
6
5
4
61
13
3,2
3
Combinatorial joining
Combinatorial VJD
65 × 27 × 6 =
40 × 5 =
30 × 4 =
70 × 61 =
52 × 2 × 13 =
12 × 3 = 36
4×3×3=
1.0 × 104
1.3 × 103
combinations
200
120
~430
36
D segments read in
Rarely
--
--
--
Often
--
--
3 frames
Joints with N and P
2
(1)
2
1
??
??
nucleotides
1.0 × 104 × 320 = 3.2 × 106
430 × 1.2 × 103 = 5.8 × 106
36 × 36 = 1.3 × 103
V gene pairs
~3 × 107
~2 × 1011
Junctional diversity
???
~1014
~1018
Total diversity
???
Source: Data from Janeway et al. (1999) pp. 62, 93, 158.
receptor interacts with both the peptide and the MHC mol-
Cytotoxic T Cells
ecule (Fig. 10.5). The T cell cannot recognize the peptide
The majority of CD8+ T cells are or become CTLs,
alone or even recognize the peptide presented by the wrong
although a minority of CD4+ T cells are also CTLs. CD8+
MHC molecule. Similarly, the T cell cannot recognize a
T cells are class I MHC restricted, as described. Because
class I or class II MHC molecule with the wrong peptide
class I MHC molecules are expressed on most mammalian
in its cleft. The discovery of this requirement for dual rec-
cells, the major exception being neurons and, in humans, red
ognition, which was made using a viral system, resulted
blood cells, which express little or no class I MHC, most
in a Nobel Prize for Doherty and Zinkernagel (Table 1.1).
cells in an individual are capable of presenting peptides to
Such a requirement for multiple interactions is a recur-
T cells in a class I MHC context.
ring theme in the immune system, and it has evolved
The peptides presented by class I MHC are derived from
because this potent and potentially harmful system must
intracellular proteins and represent a sampling of all proteins
be carefully regulated.
being synthesized within the cell. The pathway involved is
The T-cell receptor is part of a complex containing
illustrated in Fig. 10.6. The peptides are generated by prote-
accessory molecules that are required for the function of the
olysis of intracellular proteins by an enzyme system referred
receptor. Two such molecules are CD4 and CD8, and mature
to as the proteasome. The proteasome is a large complex,
T cells possess either CD4 or CD8 (but not both). CD8 con-
possessing many subunits, that is present in the cytoplasm. It
tains one Ig domain attached to a stalk region, whereas CD4
possesses ATP-dependent proteolytic activity and is the major
contains four Ig domains (Fig. 10.5). CD8+ T cells recog-
cellular proteolytic site other than the lysosome. In addition
nize peptides presented by class I MHC (Fig. 10.5A). CD4+
to its function in the immune response, the proteasome is
T cells, in contrast, recognize peptide epitopes presented
important for turnover of many proteins within the cell and
in the context of class II MHC (Fig. 10.5B). CD8 or CD4
for degradation of misfolded proteins. Peptides resulting from
interacts with constant regions of class I or class II MHC
degradation of intracellular proteins are actively secreted, in
molecules, respectively, and increases the binding affinity
a process that requires hydrolysis of ATP, into the lumen of
of the T cell for its cognate MHCpeptide complex by about
the endoplasmic reticulum (ER) by a transporter called TAP
100-fold. Class I and class II MHC molecules acquire the
(transporter associated with antigen presentation). TAP is
peptides that they present in fundamentally different ways
encoded in the MHC and consists of a heterodimer anchored
and are components of two different responses to infection
in membranes of the ER.
by microorganisms
Antigen-presenting cell
Almost any host cell
S
S
S
S
S
S
S
S
S
S
S
S
S
S
CD8
MHC Class I
MHC Class II
A
dimer
SS
S
S
S
Antigenic
Antigenic
S
peptide
peptide
S
S
S
S
S
CD4
S
S
S
S
S
a
b
a
b
TCR
chain
chain
chain TCR
chain
S
S
S
S
S
S
S
S
S
S
SS
SS
COOH
COOH
COOH
COOH
+
+
Cytotoxic CD8 T cell
CD4 Helper T cell
A
B
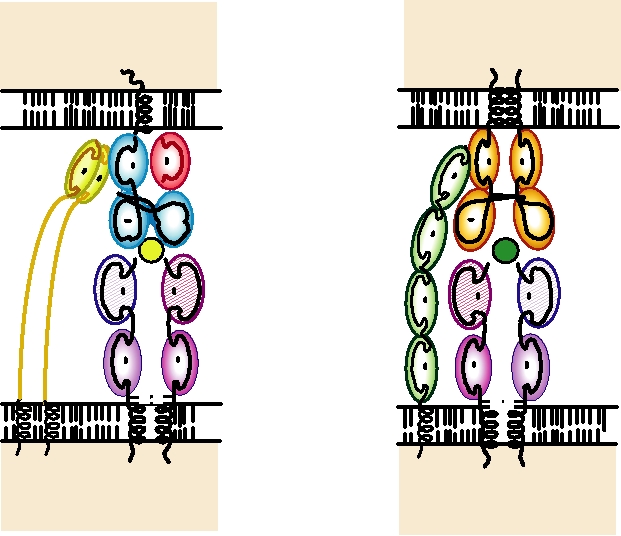
FIGURE 10.5
(A) Interaction of the TCR on a cytotoxic CD8+ T cell with an MHC class I molecule complexed with
an antigenic peptide on almost any cell. The TCR interacts with both the peptide and with the MHC molecules. The CD8
homodimer interacts with a conserved region of the MHC α3 domain. (B) Interaction of the TCR on a CD4+ helper T cell
with an MHC class II molecule complexed with an antigenic peptide on the antigen-presenting cell. The TCR interacts
with both the peptide and with the MHC molecule. The membrane distal domain of CD4 recognizes a conserved region
of the MHC β2 domain. Adapted from Chen and Alt (1997) pp. 344 and 345; and data from Kuby (1997) pp. 275277.
Proteins secreted into the lumen of the ER during syn-
proteins including the two subunits of TAP (TAP1 and
thesis are also sampled. There is a pathway that recycles
TAP2), a transmembrane glycoprotein called tapasin, the
lumenal proteins back to the cytoplasm. This pathway may
soluble chaperone calreticulin, and thiol oxidoreductase
serve to rid the ER of misfolded proteins as well as enabling
Erp57. Binding of peptide stabilizes the class I molecule and
the sampling of proteins destined for the plasma membrane
facilitates its release and transport to the cell surface. Class
or other intracellular organelles. On reentry into the cyto-
I MHC that is transported without a peptide is unstable at
plasm, a cellular glyconase removes carbohydrates from
37°C and is degraded.
glycoproteins, and the protein backbone is degraded by the
The end result is that class I MHC presents a random
proteasome. Thus, viral proteins that are inserted into the
sampling of peptides derived from proteins being synthe-
lumen of the ER during synthesis, such as glycoproteins used
sized within the cell for inspection at the cell surface by any
to assemble progeny virions, are also sampled by the
T cell that may be in the vicinity. The peptides bound to a
proteasomeTAP pathway.
single isoform of class I MHC molecules present on the sur-
A peptide delivered to the lumen of the ER by TAP can be
face of cells in culture have been analyzed by very sensitive
bound by a class I MHC molecule if it has the right anchor
techniques. More than 10,000 different peptides, present at
residues. Delivery of the peptide to the MHC is a complex
2 to 4000 copies per cell, were identified. This great diver-
process that ensures that the MHC receives a high-affinity
sity of peptides consists mostly of self-peptides, but peptides
peptide. The peptide can be shortened to the proper length
derived from intracellular viruses or other intracellular path-
(810 residues) by a protease called ERAAP if it is too long.
ogens will be represented if the cell is infected. If a patrolling
The MHC heavy chain is bound by the membrane-associated
T cell has a receptor that binds specifically to a peptide being
chaperone calnexin upon synthesis and folds into its proper
presented by the class I MHC on another cell, the T cell may
conformation. Calnexin then dissociates, β2 microglobulin
become activated and may proliferate. Once activated, CTLs
associates with the heavy chain, and the MHC is delivered
kill cells that present the epitope they recognize. In different
to the peptide-loading complex, which consists of several
assays, the number of MHCpeptide complexes required for
External antigen or
Infected cell
pathogen
or
Viral protein
synthesized in
the cell
a
A
NUCLEUS
Proteasome
Acidic vesicle
b
B
C
c
TAP
D
ER
d
(Invariant
chain
e
peptide)
E
MHC Class I
MHC Class II
FIGURE 10.6 Antigen processing by the MHC. Both class I and class II pathways are shown. On the left, a viral
protein is synthesized in the cell (a) degraded (b) and viral peptides are bound by TAP in the ER and transported into the ER
lumen, where they are bound by MHC class I molecules (c), MHC containing bound peptide is transported by cellular
vesicles through the Golgi apparatus (d) to the cell surface (e) where it is expressed for inspection by T cells. On the
right, an external antigen or pathogen is endocytosed into the cell (A) and is degraded in an acidic vesicle (B). Class II
molecules are synthesized and imported into the ER (C) with a trimer of an invariant chain peptide in the binding site, and
transported to the trans-Golgi (D) where the invariant chain is lost. The Golgi vesicle fuses with the endosome containing
the antigenic peptide (E), and the peptideMHC class II complex is transported to the cell surface. Adapted from Fields
et al. (1996) p. 351.
recognition by a CTL has been estimated to be between one
Inside the APC, the viral antigen may enter the cytoplasm
and several hundred, and may depend on the affinity of bind-
and be processed by the methods described earlier, or may
ing of the MHCpeptide target by the T-cell receptor as well
be processed and bound by MHC class I within the endo-
as the state of activation of the T cell.
somal compartment. Activation of a T cell by an APC
The activation of a T cell is a multistage process. Naļve
requires not only the recognition of the cognate antigen in
T cells are activated to become effector cells by interaction
a class I context, but also that additional immunostimula-
with professional antigen presenting cells (APCs), usually
tory signals be present. For example, if Toll-like receptors,
macrophages or dendritic cells, that present antigen in the
described later, have been activated in the APC, it expresses
context of class I or class II MHC. APCs can present anti-
additional proteins that enable it to activate the T cell. In the
gens in a class I context that are synthesized within the cell
absence of such prior activation of the APC, it will tolerize
and processed as described before, but they can also present
a T cell rather than activating it, illustrating another layer of
antigens in a class I context that they acquire from the exter-
control to avoid possible autoimmune responses.
nal environment (called cross-presentation). Cross-presenta-
Proliferation of activated CTLs requires further stimula-
tion allows APCs to respond to a viral antigen, for example,
tion by cytokines such as interleukin-2 (IL-2). The source of
even if the virus does not replicate within the APC. These
IL-2 is usually a class of T-helper (TH) cells (most TH cells are
CD4+, as described later). Activated TH-1 cells secrete IL-2 as
antigens may arise from the dissolution of an infected cell
well as tumor necrosis factor β (TNF-β), interferon γ (IFN-γ),
that dies, and enter the APC by phagocytosis or pinocytosis.
and other cytokines. Proliferation of T cells that recognize a
are released into the circulation to patrol for cells that are
specific peptide derived from a viral protein means that a vig-
infected by viruses or other intracellular pathogens.
orous CTL response against an invading pathogen ensues.
A second level of control that reduces the incidence of
CTLs kill target cells by inducing apoptosis, a cell sui-
reaction against self lies at the level of cytokine induction.
cide pathway described in a later section of this chapter.
Virus infection or infection by other parasites activate innate
One of three different mechanisms is used to induce apop-
response elements, of which Toll-like receptors are the
tosis. These mechanisms are listed in outline form here but
best known, that result in the production of inflammatory
are described in more detail when the apoptotic pathway
cytokines. Cytokines such as IL-2 or IFN are required as a
is considered. In one mechanism, the T cell releases the
second signal for CTL activation, and IFNs also upregulate
contents of granules, which contain perforins and proteases
the presentation of antigens by MHC molecules as well as
among other components, into the target cell. Perforins
other aspects of the immune response. Thus, the inflamma-
form pore structures in the plasma membrane of the target
tory response makes it more likely that T cells will respond
cell that allow ions to leach out of the cell, and the pro-
to antigens that they recognize. As described before, APCs
teases participate in the activation of cell pathways leading
may tolerize a T cell to an antigen rather than activating
to apoptosis. In a second mechanism, apoptosis is induced
it if the appropriate immunostimulatory factors are not
by triggering the Fas death receptor on the surface of the
present, another layer of control to prevent autoimmunity.
target cell. These two mechanisms lead to cell death within
Furthermore, once activated, CTLs undergo apoptosis if the
/ 6 hours. A third mechanism utilizes the TNF-α death
cytokine signals are no longer present, thus damping out any
pathway and is a slower process, leading to cell death in
autoimmune responses that might occur.
1824 hours. Killing of target cells is a drastic response
Killing of virus-infected cells by CTLs in an effort to
and the CTL pathway is directed toward eliminating inter-
eradicate viral infection relies on the ability of the cells of
nal pathogens, usually viruses. Because early proteins
most organs to regenerate from progenitor cells. In this con-
encoded by the virus can be sampled by the MHCT-cell
text it makes sense that neuronal cells express only low lev-
receptor pathway as well as late proteins, it is possible for
els of class I MHC. These cells are terminally differentiated
a T cell to kill a virus-infected cell before it has time to
and nondividing, and if killed by a CTL they are unable to
synthesize much progeny virus. Many viruses counter this
regenerate.
pathway by interfering with the ability of an infected cell
Although the ability of CTLs to kill virus-infected cells
to express class I MHC at its surface, as described later in
is well established, recent findings indicate that CTLs, as
this chapter.
well as other activated cells of the immune system, may
Although three killing mechanisms are used by differ-
also use noncytolytic means to control and clear many
ent CTLs, they are not redundant. Mice that lack the per-
virus infections. This control is thought to be achieved
by the secretion of cytokines such as IFN-γ and TNF-α.
forin gene are unable to control infection by lymphocytic
choriomeningitis virus and half die within a month of infec-
Dengue virus infection in the brains of mice is one exam-
tion. This virus is not pathogenic in normal mice or even
ple in which noncytolytic clearance appears to be impor-
in immunocompromised mice. Thus death must result from
tant. During dengue virus infection of neurons, CTLs are
immunopathology caused by an unbalanced or incomplete
actively recruited into the brain and are essential for the
T-cell response.
clearance of virus in immunized mice, at least under some
Since the class I pathway presents peptides derived from
conditions. Neurons are immunologically privileged, as
self as well as from viruses that may have infected the cell,
noted before, and noncytolytic mechanisms of control are
how then do the CTLs know not to kill cells expressing self-
important. Hepatitis B virus infection of hepatocytes is
antigens? The answer lies in part in the selection of an appro-
another system in which there is evidence that noncytolytic
priate repertoire of T cells. T cells bearing T-cell receptors
control is important, even though hepatocytes do regener-
recognizing many different possible peptide antigens arise
ate and killing of infected hepatocytes does occur, causing
by random combinatorial joining and diversity-inducing
the symptoms of hepatitis. Other examples are also known.
processes. T cells undergo their early differentiation in the
Noncytolytic clearance does not appear to be universal,
thymus, where selection occurs. Only T cells that express
however. It appears to be possible only in some tissues and
a T-cell receptor capable of recognizing class I MHC (or
for some viruses.
class II MHC) bearing a peptide are selected (called posi-
tive selection); T cells that do not express an appropriate
T-Helper Cells
receptor die. However, if such a T-cell receptor has a high
Most CD4+ T cells are helper cells. Some CD8+ T cells
affinity for self-peptides present in the thymus, then that T
are also helper cells, but most helper cells are CD4+. CD4+ T
cell also dies (called negative selection). In this process of
selection only about 2% of T cells survive and most of these
cells recognize peptides presented in the context of class II
encode receptors that recognize nonself antigens. These T cells
MHC molecules--they are referred to as class IIrestricted
cells (see Fig. 10.5). Class II MHC molecules, unlike class I,
make antibody (see later). It is the disappearance of TH cells
are present on only a restricted set of cells within the organism
during HIV infection that leads to the symptoms of AIDS.
and are most abundant on B cells, macrophages, dendritic
TH cells are not homogeneous. Different TH cells secret
cells, and, in humans, activated T cells, that is, cells of the
different panels of cytokines and have different functions in
immune system itself.
the immune response. Two main types have been recognized,
The peptides presented by class II MHC molecules are
which perhaps represent extremes in function. TH-1 cells are
derived from extracellular proteins, and thus these MHC
highly effective for CTL activation and function in the cel-
molecules sample the extracellular environment. Proteins,
lular immune pathway. TH-2 cells are optimal for the activa-
whole viruses, or other microorganisms are taken up by
tion of B cells and function in the humoral immune pathway.
antigen-presenting cells and degraded within intracellu-
They secrete IL-4, IL-5, IL-6, and IL-10. TH-1 and TH-2 cells
lar organelles. Peptides derived from these sources can be
can be mutually antagonistic. The cytokines secreted by one
bound by class II MHC molecules being transported to the
suppress the other, and a balanced immune response often
cell surface. The process of producing a peptide and trans-
requires a balanced activation of these two classes of help-
ferring it to a class II MHC molecule is complicated, as is
ers. TH-2 cells usually deliver their cytokine signals directly
the case for class I MHC, but will not be described here since
to the B cells that they help, following cellcell contact. TH-1
viruses are not known to interfere with the class II pathway,
cells, in contrast, do not deliver their signals directly to the
whereas they do interfere with the class I pathway. The pep-
T cells that they help.
tides derived from this pathway are kept separate from pep-
tides generated through the proteasomeTAP pathway, and
B Cells and Secretion of Antibodies
the end result is that the class II pathway presents peptides
derived from the external environment, whereas the class I
B cells, so named because they are derived from the bone
pathway normally presents peptides derived from the intrac-
marrow in mammals, secrete antibodies, which are members
ellular environment (Fig. 10.6) (but see the previous discus-
of the Ig superfamily. The essential subunit of an antibody is
sion of cross-presentation).
a heterodimer of a heavy (H) chain (which has four or five Ig
Professional APCs, which may be macrophages, B cells,
domains) and a light (L) chain (which has two Ig domains).
or dendritic cells, present peptides to T cells. They also
This subunit is always present as a dimer in which two H-L
express accessory proteins that deliver a second signal to the
heterodimers are linked through the H chains. The structure
T cell that is required for its activation. When a TH cell is acti-
of a light chain, as determined by X-ray crystallography, is
vated by the interaction of its receptor with its target peptide
shown in Fig. 10.7. This structure illustrates the Ig fold that
presented by class II MHC, and the appropriate second sig-
is common to all Ig domains.
nals are present, as described before, the cell proliferates and
The five classes of antibodies are illustrated in Fig. 10.8.
secretes cytokines that are important for eliciting an immune
An IgG molecule consists of a dimer of H-L heterodimers in
which the H chain is of the γ class. IgD and IgE antibodies
response. Activated TH cells are essential for any immune
response, whether CTLs to kill infected cells or B cells to
are also dimers of H-L heterodimers, but in this case the H
CL domain
VL domain
Loops
β strands
C-terminus
N-terminus
CDRs
Disulfide bond
FIGURE 10.7
Ribbon diagram of an immunoglobulin light chain depicting the immunoglobulin-fold structure of its
variable and constant domains. Two β-pleated sheets in each domain (colored in red and pink in the constant domain and
yellow and brown in the variable domain) are held together by hydrophobic interactions and a single disulfide bond (dark
bar). The hypervariable regions also known as complementarity determining regions (CDRs), shown in blue, form part of
the antigen-binding site. Adapted from Kuby (1997) p. 113.
IgG
IgD
IgE
The two Ig domains of the L chain are called V (for variable)
VL
VL
VL
and C (for constant) and between these two domains is a J
VH CL
VH CL
VH CL
(for joining) domain. All three domains are encoded sepa-
Cg 1
Ce 1
Cd 1
SS
rately in the genome. There are multiple copies of V, J, and
SS
SS
SS
Ce 2
C in the genome (Table 10.1), and these gene segments are
Cg 2
Cd 2
SS
Ce 3
polymorphic in the population. The light chain genes fall into
Cg 3
Cd 3
two different families, called κ and λ (Table 10.1), which are
Ce 4
encoded on two different chromosomes in humans. During
maturation of a B cell, a V-gene segment, a J-gene segment,
IgA Dimer
IgM Pentamer
and a C region of the light chain are brought into juxta-
VL
VL
position to one another, as illustrated in the top panel of
VH CL
VH CL
Fig. 10.10. In this process, a V-gene segment is fused to a
Cm 1
Ca 1
J-gene segment by deleting the intervening DNA. The C
SS
SS
Cm 2
region is brought into play by RNA splicing: transcription of
SS
Ca 2
the VJ region continues through the C region, and splicing of
Cm 3
S
SS
S
Ca 3
the pre-mRNA joins the J region to the C region.
Cm 4
SS
The heavy chain, which is the first to rearrange, is formed
S S
SS
S S
by a similar sequence of events, but in this case there is an
SS
SS
S
S
additional gene segment D (for diversity) that introduces
S
S
additional diversity in the recombination process. As for the
SS
SS
SS
light chain, there are multiple copies of V, D, and J in the
SS
genome (Table 10.1), and the population is polymorphic for
these gene segments. During B-cell maturation, a D-gene
segment is first joined to a J segment, and the DJ segment is
then joined to a V segment (bottom panel of Fig. 10.10).
Recombinational rearrangements to form the antigen-
md
g
ea
binding site occur only during the maturation of the B cell.
Once a B cell is mature, no further rearrangements occur in
FIGURE 10.8
Structure of the different classes of secreted immuno-
this region, although during an immune response the anti-
globulin molecules. Note that IgM and IgA are secreted as pentamers and
dimers, respectively, linked by a J-chain. Adapted from J. Gally (1973).
gen-binding site is subjected to hypermutation in germinal
IgG, IgA, and IgD heavy chains have four domains and a hinge region; IgE
centers, as described later.
and IgM lack the hinge. Not shown are intrachain disulfide bonds and bonds
The many combinatorial possibilities of V and J light-
linking the light and heavy chains.
chain gene segments, and of V, D, and J heavy-chain gene
segments, lead to the possible production of a very large
number of light chains and heavy chains (Table 10.1). In
chains are of the δ or ε class, respectively. IgA antibodies
addition, joining V with J, or joining V, D, and J, is impre-
contain four H-L heterodimers (it consists of a dimer of the
cise, leading to additional diversity. Finally, joining an L
dimeric unit, as shown). In this case, the H chain is of the α
chain with an H chain to form the heterodimer generates still
class. IgM contains 10 H-L heterodimers (it is a pentamer of
more possible antigen recognition sites, since the antibody
dimeric units) formed with µ H chains.
recognition site is formed by the V regions of both the
A more detailed representation of an IgG molecule is
H and the L chains. The total number of possible combinations
shown in Fig. 10.9. The terminal domains of both H and
is very large (Table 10.1). It is important to note that any
L chains are variable. Within the variable domains, there
individual B cell usually produces only one H chain and one
are regions that are more variable than other regions, called
L chain, and thus each B cell produces only one antibody
hypervariable regions, also known as complementarity-
recognition site.
determining regions (CDRs) (see Fig. 10.7). The combining
site of the antibody, the region that specifically binds to an
Activation of B Cells
antigen recognized by the antibody, is formed by the vari-
able regions of both the H and L chains.
During maturation of B cells, a large population of cells
results, each of which has one antibody-combining site. The
theoretical repertoire, how many different types of B cells
Formation of Light and Heavy Chains
could conceivably be produced using the known mecha-
nisms that are active during B-cell development, is thought
The L and H chains of antibody molecules are formed in
to be much larger than the minimal estimate of 1014 shown
a process that is similar to that used to form T-cell receptors.
Variable regions
Antigen-binding
domain
VL
S
S
S
S
S
S
S
S
VH
CL
S
S
S
S
S
S
S
CH1
S
S
Light chain
Heavy chain
S
S
S
variable
variable
Heavy Chains
S
S
region
region
S
S
Constant
CDR1
S
CDR1
S
H
s
S
VH
VL
or
Variable
CDR2
CDR2
s
S
Hypervariable (CDR's)
S
S
JL
DH
CH2
Hinge
S
S
JH
Effector
function
Light Chains
domain
S
S
Constant
CH3
S
S
Variable
Hypervariable (CDR's)
FIGURE 10.9 Diagrammatic representation of an IgG molecule. Each antibody molecule is composed of two heavy
chains (which contain four domains, each consisting of an Ig fold like that shown in Fig. 10.7) and two light chains, which
each have two Ig domains. The distal domains of both the light and heavy chains are the variable regions, composed of
interspersed framework regions and hypervariable regions (diagonal shading) known as complementarity-determining
regions or CDRs. The right part of the figure illustrates the role of the V, D, and J gene segments in encoding CDR1, 2,
and 3 regions of Ig variable region genes. Adapted from Chen and Alt (1997) pp. 340 and 345.
in Table 10.1. In humans there may be 1010 B cells with
that recognize this peptide will secrete cytokines that stimu-
differing specificities circulating at any time, and it is esti-
late the B cell to proliferate and secrete antibodies. Note that
mated that about 1 in 105 cells produces an antibody that will
the peptide displayed by the class II molecule does not have
bind, with differing affinities, to any particular antigen that
to be related to the epitope recognized by the antibody dis-
is being examined.
played on the B-cell surface. It may, in fact, be derived from
The antibody molecule is first produced as an integral
an entirely different protein. Thus, while T cells respond to
membrane protein that is displayed on the surface of the
peptide epitopes, the antibody molecules can recognize much
B cell, anchored through a membrane-spanning region and
more complex antigens, such as whole proteins or viruses
containing an intracellular cytoplasmic domain. If the anti-
or even nonprotein antigens like carbohydrates. The pro-
body displayed on the surface of the cell binds antigen, the
tein epitopes recognized by antibodies are most often what
B cell is activated. If the cell receives a second signal from
are called conformational or nonlinear epitopes, which are
a TH-2 cell, it proliferates to form cells that secrete antibody.
formed by residues physically located at different places in
Memory cells also arise that serve to protect the organism
the linear sequence of a protein but which form a contiguous
from future infection by the same pathogen.
surface in the protein after it folds into its three-dimensional
The TH-2 cell signal may be delivered to the B cell either
conformation. Such discontinuous epitopes are destroyed if
through a specific pathway or through a nonspecific path-
the protein is denatured and the different components of the
way. Antigen, which could be in the form of a whole virus or
epitope separated from one another. A certain fraction of
in the form of a protein, that is bound to antibody present on
antibodies, however, recognize continuous epitopes, which
the surface of the B cell can be internalized by the B cell and
are formed by a linear sequence of amino acids present in
degraded by the MHC class II antigen-processing pathway.
the protein.
Peptides derived from the degraded virus or protein can then
In addition to specific activation of B cells by T-helper
be presented on the surface of the B cell in the context of
cells, B cells can also be activated through area stimu-
class II MHC molecules. Class IIrestricted T-helper cells
lation. If a B cell is in the vicinity of T-helper cells that
Light Chain
Jk
Cκ
LVk1
LVk1
LVkn
Germline k-Chain DNA
V-J Joining
Jk
Ck
VkJk
LVk1
Rearranged k-Chain DNA
Transcription, RNA Splicing, Polyadenylation
Ck
L VJ
5'
3'
Light Chain (k) mRNA
An
Cap
Translation
Light Chain (k) Protein
Ck
Vk
VH Cm1 Cm2 Cm3 Cm4
Heavy Chain (m) Protein
5
IgM
Translation
LV DJ Cm
5'
3'
Heavy Chain (m) mRNA
An
Cap
Transcription, RNA Splicing, Polyadenylation
LVH179 V DJ J
L VH1
Rearranged Η-Chain DNA
H
V-DJ Joining
DH1 DH6 DJ JH
VH180 VHn
LVH1
D-J Joining
Germline Η-Chain DNA
Cm
Cd
Cg3 Cg1 Cg2b Cg2a Ce
Ca
VHn DH1 DH7 DH13
LVH1
JH
Heavy Chain
FIGURE 10.10 Formation of the IgM heavy and light chains. (Top) Illustration of the germ-line genes encoding κ
immunoglobulin light chains encoded on human chromosome 2. The first line shows the germ-line genes, and the next
line illustrates the B-cell genes after VJ recombination. The next line down shows the RNA transcript after splicing to
join the VJ region to the C region. This mRNA is translated by cytoplasmic ribosomes into the light chain, which then
combines with a heavy chain. The leader, L, is translated into a signal sequence that is removed posttranslationally.
(A similar series of rearrangements occurs among the gene segments for the λ light chains encoded on human chromosome
22.) (Bottom) A comparable illustration of the heavy chain genes in the germ line and in the B cell, after two rounds of
rearrangement known as "DJ joining," and "VDJ joining." The final IgM molecule is a pentamer held together with
disulfide bonds and a "J chain" which links the Fc regions. Adapted from Kuby (1997) Figures 7.4 and 7.5 on pp. 172
and 173.
are releasing cytokines to activate B cells, it may also be
antibodies. The first antibodies secreted are IgM, whose
stimulated. The importance of area stimulation, and the fre-
structure is illustrated in Fig. 10.8. IgM antibodies, which
quency with which it occurs, in the context of fighting off a
circulate in the blood, can be detected as early as a few days
viral infection is not clear. In many cases of viral infection,
after virus infection. Their production quickly wanes and
a generalized and active inflammatory response occurs that
over a period of weeks or months the concentration of IgM
involves the release of many cytokines, and in which many
in the blood decreases to very low or undetectable levels, as
different antigens are being presented. Area stimulation
illustrated schematically in Fig. 10.11. Thus, the presence of
could be important in developing a rapid response during
IgM antibodies specific for a virus is usually a sign of acute,
such events. However, in such a process antibodies against
or at least very recent, infection.
self might also be produced, and such processes must be
During further maturation of the B cell, class switching
controlled.
occurs, as illustrated in Fig. 10.12. Recombinational events
in the heavy-chain region lead to the substitution of the
IgG, IgE, or IgA heavy chain for that of IgM. Homologous
Secretion of Antibodies
recombination within the intron just downstream of the J
A B cell stimulated by exposure to its cognate antigen and
gene results in deletion of the intervening DNA such that the
by help from a TH-2 cell proliferates and begins to secrete
active VDJ gene is brought into contact with the C region
IgA) or directly (e.g., IgM to IgE without passing through
100
Total
an IgG phase). The situation in the human chromosome is
more complicated (Fig. 10.12). There are two α genes, the
first of which lies between two of the four γ genes. Thus,
10
Secondary
Primary
there are more ways to switch from one class of antibody
Response
Response
to another.
1
Production of IgG (or of IgE or IgA) thus occurs later
Total
after infection (illustrated schematically in Fig. 10.11). At
IgG
least 2 weeks are required before there is production of
0.1
IgM
IgG
large amounts of IgG. Once a B cell begins to make IgG,
IgM
it is no longer able to make IgM because the gene encod-
ing the M heavy chain (Cµ in Fig. 10.12) has been deleted.
Note that the antigen-combining site of an antibody is not
1 Antigen
2 Antigen
changed by class switching because the V region of the H
Time after immunization
chain is not involved. However, hypermutation in the com-
FIGURE 10.11 Time course of development of circulating antibodies
bining site to increase the affinity of the antibody for the
after primary and secondary immunizations. No timescale has been shown,
antigen, which occurs in germinal bodies, is associated with
since the actual results vary with antigen, adjuvant, site of injection, and
class switching because a deaminase that is induced upon
animal species. From Kuby (1997) Figure 16.19 on p. 398.
activation of the B cell is involved in both class switching
and hypermutation.
IgG circulates in the blood and therefore very many cells
of a different class of heavy chain. The order of heavy-chain
exposed to blood and blood products are exposed to IgG.
genes in the mouse chromosome is µδγεα, and class switch-
IgG lasts only 2030 days in the blood, but B cells continue
ing can happen either sequentially (IgM to IgG to IgE to
ΨCε
Cµ
Cγ 1
Cγ 3
Cγ 2
Cα 2
Cα 1
Cδ
Cγ 4
Cε
VDJ
H-Chain DNA
Sµ
Sα 1
Sγ 3
Sγ 1
Sγ 2
Sγ 4
Sε
Sα 2
Recombination between Sµ and Sγ1
Cγ 1 Ψ C ε
Cγ 2
Cα 2
Cα 1
Cγ 4
Cε
VDJ
Class-switched
Sα 1
H-Chain DNA
Sγ 4
Sε
Sα 2
SS 2
γ
Sγ 1
Recombination between Sγ 1 and Sε
Transcription,
Splicing,
Polyadenylation
VDJJH
Cα 2
Cε
γ1
I
Sα 2
5
Cγ 1
5
VDJ
Transcription,
IgG1 mRNA
Cap
An
Splicing,
Polyadenylation
T
3
3
Cε
VDJ
ranslation
gE mRNA
Cap
An
Translation
IgG Heavy Chains
IgE Heavy Chains
FIGURE 10.12
Immunoglobulin class switching to produce heavy chains for IgG and IgE, following initial
rearrangements to produce IgM. Switch sites, designated "S", are located upstream of each CH gene except Cδ. In
humans there is only one allele for Cµ, Cδ, and Cε, but there are two alleles for Cα, designated α1 and α2 and four alleles
for Cγ, designated γ1, γ2, γ3, and γ4, which differ somewhat in sequence. In addition there is a pseudogene related to Cε.
Because there is no switch site for Cδ, IgD is produced only in conjunction with IgM, by alternative mRNA splicing.
Adapted from Kuby (1997) Figure 7.17 on p. 185; and Janeway et al. (2004) Figure 4.18.
to produce antibody for long periods of time, and although
Antibodies may bind to a virus and neutralize its infec-
production wanes with time, IgG remains circulating in the
tivity, and such neutralizing antibodies are thought to be of
blood for years or decades. Because of this, and the ease
critical importance in controlling virus infection. However,
of obtaining blood samples, IgG has been extensively used
other antibodies may bind to a virus without inactivating it
to monitor past exposure to different pathogens and the
and such nonneutralizing antibodies can also be protective.
immune status of individuals for any particular pathogen.
There are several mechanisms by which nonneutralizing
As illustrated in Fig. 10.11, on a second exposure to an anti-
antibodies may protect. Aggregation of virions by antibodies
gen, IgG concentrations rise dramatically and remain in the
leads to a reduction in the total number of infectious viruses.
circulation at much higher levels. IgM concentrations do not
The maturation of enveloped viruses by budding through the
show this anamnestic effect.
cell plasma membrane can be inhibited by the binding of
Because of its widespread circulation within the body, its
antibodies to viral proteins present on the surface of the cell.
high concentrations, and the anamnestic effect that occurs
Antibody-dependent lysis of an infected cell can occur by
on secondary exposure, IgG is important in the control of
a complement-mediated pathway or by a natural killer cell
viral diseases. It is also important in protecting the fetus and
pathway called antibody-dependent cell-mediated cytotox-
the very young. IgG crosses the placenta during pregnancy
icity. These pathways are triggered by the binding of anti-
and is present in fetal blood. This transfers maternal immu-
body to viral protein expressed on the cell surface. Binding
nity to the fetus, and this immunity lasts for the first few
of antibody or of certain components of complement to a
months of postnatal life.
virus can also increase the rate of phagocytosis by macro-
IgA is also present in the blood, but its importance lies in
phages, which results in the destruction of the antigen, a
the fact that it is secreted. It is present on mucosal surfaces
process called opsonization. Complement and natural killer
where it helps prevent viral diseases such as those caused
cells, which are components of innate immunity as well as
by rhinoviruses or influenza viruses in the respiratory tract
of acquired immunity, are described later. Other mechanisms
or by rotaviruses or enteroviruses in the intestinal tract. It is
that result in protection by antibodies also exist.
present in secretions such as tears, saliva, and genital tract
secretions, where it plays an antiparasite role. Because it is
Immunologic Memory
present in milk, it also serves to transfer maternal immunity
to the gut of the infant.
In the course of the B-cell response, memory B cells
IgE is important for control of infection by multicellular
are formed. Memory B cells persist after the antigen disap-
parasites. It can bind to mast cells via specific receptors and
pears from the body, and are primed to react quickly and
cause an inflammatory response, leading to the destruction
vigorously on renewed stimulation by the cognate antigen.
of parasites. It is IgE that produces allergic symptoms that
Renewed activity of memory B cells still requires T-helper
occur when pollen granules or mites or other comparatively
cell stimulation, but the secondary response leads to imme-
large particles are recognized by IgE, producing an inflam-
diate production of antibody of high affinity, because there
matory response.
is no need for a long maturation process, and is so vigor-
IgD is present in a membrane-bound form at the surface
ous that it results in the production of much larger amounts
of immature B cells, along with IgM, where it helps in the
of antibody than are produced during a primary response
activation of the cell on exposure to antigen as described
(Fig. 10.11).
before. It is also present in very small amounts in the blood.
Memory T cells are also generated in the course of an
There is no class switching mechanism to express IgD and it
immune response. After expansion of T-cell clones following
is only produced in combination with IgM by means of dif-
stimulation by the cognate antigen, most activated T cells die
ferential splicing of mRNAs.
by apoptosis when no longer stimulated by the presence of
Although the B-cell repertoire first arises by combinato-
antigen and cytokines. Immunologic memory remains, how-
rial joining of the V and J light-chain gene segments and of V,
ever. It had been widely thought that such memory requires
D, and J heavy-chain gene segments, the antibody response
a continuous supply of antigen with which T cells interact.
is fine-tuned once a B cell has been activated. There is an
This antigen might be present because the virus establishes a
error-inducing mechanism during B-cell replication in which
chronic infection, or antigen might be sequestered in regions
the genes encoding the V segments of both heavy and light
of the body and made available over an extended period of
chains undergo hypermutation. This activity takes place in
time. It was thought even possible that T cells continued to
germinal centers of the lymph node and there is concurrent
be stimulated because of cross-reactivity with self antigens.
selection for B cells that bind more tightly to the antigen. Over
Data have now accumulated, however, that memory T cells
a period of time B cells are selected that bind to the antigen
exist in a quiescent state for long periods of time and are
with higher and higher affinity. Hypermutation in germinal
capable of rapid reactivation on renewed exposure to anti-
centers has been postulated to play a role in the development
gen. Thus, memory in T cells is now thought to be similar to
of Burkitt's lymphoma, as described in Chapter 7.
memory in B cells.
It is the existence of memory cells that renders an ani-
other than cell lysis. Some products enhance the neutrali-
mal immune to the virus or other pathogen that first evoked
zation of viruses by antibody. By binding to virus that is
the immune response. Memory cells are primed for such a
coated with antibody, they render the virus less capable
rapid and vigorous response that the invading organism is
of binding to its receptor; cause aggregation of the virus,
stopped early during the infection process, before disease
resulting in fewer infectious units; and increase the uptake of
is established. Residual antibodies circulating in the blood
viruses by phagocytic cells (opsonization). Other molecules
or present on mucosal surfaces may even prevent infection
induce an inflammatory response, in part by inducing the
altogether (sterilizing immunity).
release of agents by mast cells and basophils, or take part in
The immune status of an individual is often tested by
the activation of B cells, or help in the clearing of immune
examining the blood for the presence of antibodies. Such
complexes.
an assay is imperfect, however. The presence of antibod-
Numerous studies have shown that complement is
ies in the blood is usually a good indication that a person is
required for an effective immune response. As one example,
immune. However, although such antibodies fade with time,
mice lacking a receptor for an early product of the comple-
in many cases a person remains immune despite the absence
ment cascade, called C3d, are unable to mount an antibody
of detectable antibodies in the blood, because memory cells
response. As a second example, depletion of complement in
that are primed to react quickly are still present.
mice infected with Sindbis virus leads to a prolonged viremia
and a more severe central nervous system disease. As a third
example, people are known who are genetically deficient for
Complement System
components of the complement pathway. Many suffer from
The complement system is composed in part of more than
immune-complex diseases as a consequence, because they
20 soluble proteins that circulate in the blood. These pro-
are unable to effectively clear immune complexes. They also
teins are activated through a proteolytic cascade to produce
suffer from an increased incidence of bacterial infections.
effector molecules that aid in the control of viral infection
or infection by other pathogens. Complement forms part of
Adaptive Immunity in the Control of
both the adaptive immune system and the innate immune
Virus Infection
system. Its activities turn antibodies into effective kill-
ers of viruses or of virus-infected cells, as well as of other
It has been conjectured that the two arms of the adaptive
pathogens, and in this role it is a component of the adaptive
immune system evolved to fight off different pathogens. The
response. Complement can also be activated by interaction
CTL response seems well adapted for the control of viral
with parasites in the absence of antibody, however, and in
infections because these pathogens replicate intracellularly,
this role it is a component of the innate responses.
but less well adapted for controlling extracellular patho-
The classical pathway of complement activation (an adap-
gens such as bacteria or protozoa. Conversely, the humoral
tive response) involves interaction of a complex of comple-
response and the associated complement system seem better
ment molecules called C1 with IgG or IgM. This could be
adapted for the control of extracellular pathogens. Consistent
IgG or IgM bound to antigen present at the surface of an
with this model, children who are deficient in the production
infected cell or a bacterium, for example. The alternative
of antibodies do not in general show an increased suscep-
pathway of complement activation (innate response) does
tibility to viral diseases but do show a marked increase in
not involve interaction with antibody, but rather requires the
susceptibility to bacterial infection. Children unable to make
deposition of a molecule called C3b on the surface of a parti-
gammaglobulin, for example, recover normally from infec-
cle, such as a parasite. Once bound, C1 or C3b interacts with
tion by measles virus and are immune to reinfection, dem-
other components of the complement system. The result
onstrating the importance of T-cell immunity in this disease.
is the activation of a cascade of proteases whose cleavage
Conversely, impairment of CTL function in children often
activities result in the formation of effector molecules. One
leads to increased frequency and severity of virus infec-
group of effector molecules forms a complex that inserts into
tions. Such findings suggest that CTLs evolved primarily to
the lipid bilayers of cell membranes and results in the lysis
deal with viral infections and remain of prime importance
of the cell. Many enveloped viruses can also be killed by
in dealing with viral infections, whereas the humoral sys-
this lytic mechanism. The system must be finely regulated so
tem evolved to deal with infections by free-living organisms
that activated components of complement are produced only
such as bacteria, protozoa, and yeast.
in response to pathogens and so that cell killing is confined
Although this model may well be correct, it is clear that
to infected cells or parasites. Control of complement activa-
humoral antibodies are also important in the control of viral
tion and action is therefore suitably complicated.
disease. Many experiments have shown that passively trans-
Other effector molecules that result from activation of
ferred antibodies alone can protect against viral infection.
complement have activities that aid in the control of viral
Further, whether reinfection by a virus results in disease or
infection or infection by other parasites by mechanisms
asymptomatic infection is often correlated with the level of
antibodies against the virus in the blood. These observations
people concerned with their appearance). This technique
are particularly relevant to the protection of an unborn or
was greatly refined about 200 years ago by Jenner, who
newborn child from viral disease. Maternal antibodies are
immunized people against smallpox with a nonhuman virus
actively introduced into the fetal bloodstream during intra-
derived from cows. Cowpox virus is antigenically related
uterine development. Such antibodies are critical for the pro-
to smallpox virus and induces immunity to smallpox, but
tection of the fetus and the newborn against viral infections
does not cause such severe disease as smallpox. This immu-
early in life before its own immune system develops. It is
nization procedure was very successful and smallpox has
also clear that neutralizing antibodies are of prime impor-
now been eradicated using modern versions of Jenner's
tance in preventing reinfection by at least some viruses, such
original vaccine. The process of using a nonhuman virus to
as influenza virus. Previous infection leading to a vigorous
induce immunity in humans against a related human virus
T-cell response directed against many of the viral proteins
has been referred to as "the Jennerian approach." Jenner's
does not protect against subsequent reinfection by vari-
use of cowpox virus to immunize against smallpox gave us
ants which are altered only in their surface glycoproteins.
the name "vaccination" and "vaccine," from the Latin word
As another example of the importance of humoral antibod-
vacca meaning cow.
ies in combating viral infections, CTL-induced cytolysis
Since Jenner's time, other approaches to vaccination have
is not very effective in the control of viral infection of the
been developed. Rather than using a nonhuman virus as a
brain. Neurons are terminally differentiated and cannot be
vaccine, it is more common to use an attenuated strain of
replaced, and express only low levels of MHC class I mol-
a virulent human virus. Attenuation has classically been
ecules. However, CTLs do appear to be important in control
achieved by passing the virus in animals or in cultured cells
of viral infections in the brain, perhaps because they secret
from animals. Passage selects for viruses better adapted to
IFN-γ when activated. Other mechanisms involving humoral
grow in the nonhuman host and often results in a virus that is
antibodies are also probably important. It has been shown in
attenuated in humans. One of the earliest vaccines to be devel-
a mouse model that humoral antibodies can cure persistently
oped in this way was a rabies vaccine developed by Pasteur
infected neurons of viral infection.
by passing the virus in rabbits. A more modern method for
Thus, a broad and varied immune response to viral infec-
producing a vaccine strain, which is still often used today,
tion is important for both the suppression of the original
was introduced by Theiler and Smith, who passaged virulent
virus infection and in evoking a status of immunity to sub-
yellow fever virus in chicken tissue and chicken cells in cul-
sequent reinfection by the virus. Experiences with vaccines
ture. After 100 passages, a marked change in virulence of the
used in humans support this idea. Some vaccines have been
virus occurred. Although the passaged virus retained its abil-
found to provoke an unbalanced response that renders sub-
ity to infect humans, it no longer caused disease. Passage of
sequent infection by the virulent virus more serious, such as
virus in tissue culture cells and selection of attenuated vari-
early vaccines against measles virus and respiratory syncy-
ants has been used to produce vaccines for measles, mumps,
tial virus, discussed elsewhere.
and rubella, among others. With modern technology, it is
now possible to introduce mutations into a viral genome
that might be expected to attenuate the virus and to test the
Vaccination against Viruses
effects of such mutations in model systems (see Chapter 11).
Although no currently licensed human vaccines have been
For most viruses, once a person has been infected and
produced in this way, it is expected that this approach will be
recovered, he or she is immune to subsequent reinfection
useful for future vaccines.
by the same virus. This is the concept behind immunization,
In a few cases it is possible to infect humans with a viru-
also called vaccination, in which a person is exposed to a
lent virus in a way that does not lead to disease. Oral vaccines
virus, either live attenuated virus or inactivated virus, or to
have been developed for adenoviruses 4 and 7 in which the
components of the virus, in order to establish the immune
virus is encapsulated in a protective coating that does not dis-
state.
solve until the virus reaches the intestine. The viruses repli-
cate in the intestine but do not produce disease, although they
Live Virus Vaccines
do induce immunity against adenovirus respiratory disease.
Live virus vaccines in general induce a more protective
Immunization has been practiced for centuries, having
and longer lasting immunity than do inactivated virus vac-
been introduced a millennium ago for smallpox. In a proc-
cines. They replicate and therefore produce large amounts
ess called variolation, less virulent strains of smallpox virus
of antigen over a period of days or weeks that continues to
were introduced into humans by intranasal inoculation.
stimulate the immune system. Furthermore, the viral anti-
The disease induced by this procedure had a lower fatal-
gens are presented in the context of the normal viral infec-
ity rate than that caused by the epidemic disease (although
tion and these vaccines induce the full range of immune
the fatality rate was still significant), and the extent of
responses, which includes production of CTLs as well as
pocking or scarring was less (which was of importance to
antibody. Live virus vaccines may be more effective than
taminated with SV40, for example, which was present in
inactivated vaccines in eradicating the wild-type virus from
the monkey kidney cells being used to prepare the vaccine.
a society. As described in Chapter 3, an inactivated poliovi-
Millions of people were unknowingly infected with SV40,
rus vaccine protects the individual from disease but it does
which fortunately appears to cause no disease in humans,
not prevent the wild-type virus from circulating. Finally, live
or at least to cause disease (some rare brain tumors) only
virus vaccines are cheaper to produce and administer than
very rarely. Finally, living viruses are often unstable, mak-
inactivated virus vaccines because a single dose containing
ing it difficult to transport vaccines over long distances
smaller amounts of (live) virus is usually sufficient to induce
and to store them so that they maintain their potency, a
immunity.
problem that is more acute in developing tropical coun-
Although they have many advantages, live virus vac-
tries.
cines also suffer from a number of potential problems.
Although there are potential difficulties, live virus vac-
Attenuating the virus sufficiently so that it does not cause
cines have many advantages and have been extremely suc-
disease while retaining its potency for inducing immunity
cessful in the control of viral diseases. Smallpox virus has
can be difficult to achieve. The human population is outbred
been eradicated, measles virus and poliovirus are on the brink
and individuals differ greatly in health status, immune com-
of eradication, and many other serious diseases have been
petence, and ability to fight off viral infections, yet a single
controlled by live virus vaccines. A partial list of currently
vaccine must be useful for all, or at least most, individu-
licensed virus vaccines, many of which use live viruses, is
als in the population. Furthermore, many viruses quickly
given in Table 10.2.
become overattenuated on passage, losing their ability to
induce immunity on infection of humans. Another problem
is the potential for reversion of the virus to virulence and
the possible virulence of the attenuated virus in normal or,
TABLE 10.2 Available Viral Vaccines
especially, immunocompromised people. In the case of the
Live attenuated
Sabin polio vaccine, about 10 vaccine-related cases of para-
virus
Killed virus
Subunit vaccines
lytic polio occurred every year in the United States due to
reversion to virulence of the virus (Fig. 3.4) before it was
Poliovirus (Sabin)
Polio (Salk)
Hepatitis B
replaced with an inactivated vaccine. Certain lots of yellow
West Nilea
Measles
Rabies
fever virus vaccine have also been found to contain partial
Human papillomavirusb
Mumps
Influenza
revertants that can cause encephalitis, especially in infants.
Rubella
Hepatitis A
Because of this, each lot of yellow fever vaccine must be
Yellow fever
Japanese encephalitis
carefully monitored for virulence and vaccination of infants
Vaccinia
Western equine
under 6 months of age is not recommended. Despite this
encephalitis
Varicellac
care, a few deaths from vaccine-induced yellow fever have
Rotavirusd
occurred very recently, which appear to be due to residual
virulence of the virus in persons with immune systems
Adenovirus (in
military recruits)
that are inadequate to handle the infection with this virus
Junin (Argentine
because of age or other reasons.
hemorrhagic fever)
Interference caused by activation of the innate immune
Influenzae
system can also cause problems. The effectiveness of a
live virus vaccine may be diminished because of interfer-
a
Recombinant vaccine on yellow fever 17D backbone in clinical trials;
ence from a preexisting infection. Interference also makes
already in use as veterinary vaccine.
it difficult (although not impossible) to immunize simul-
b
Recombinant vaccine (Gardasil) based on virus-like-particles composed
taneously against multiple viruses when using live virus
of proteins L1 and L2 approved for girls 926 years old by the FDA
vaccines. This becomes a particular problem with vac-
on June 8, 2006. Quadrivalent vaccine contains antigens from HPV16,
cines that contain multiple components, such as vaccines
HPV18, and types 6 and 11 of HPV 6.
c
Live attenuated vaccine (Zostavax) to protect adults over 60 who have
for the four serotypes of dengue virus or for four serotypes
previously had varicella from contracting shingles, approved May 25,
of rotaviruses. However, the mumpsmeaslesrubella vac-
2006.
cine contains three live, attenuated viruses and this vaccine
d
Second generation pentavalent vaccine (Rota Teq) approved by the FDA
has been very successful in controlling these three viruses
on Feb. 3, 2006.
with a single inoculation (albeit that a booster is recom-
e
New generation live vaccines composed of reassortants of previous
vaccine strains with HA and NA of current epidemic strain being
mended after 10 years or so). Problems can also arise from
developed.
the presence of adventitious infectious agents in the vac-
Source: Data for this table came from Granoff and Webster (1999)
cine, since it has not been treated with inactivating agents.
p. 1862; from Fields et al. (1996), p. 371; and from Arvin and Greenberg
Early lots of the live Sabin poliovirus vaccine were con-
(2006).
Inactivated Virus and Subunit Vaccines
Despite these problems, many successful vaccines
have been introduced that use inactivated viruses or sub-
The second general approach to vaccination is to use
units of viruses, and some of these are listed in Table 10.2.
inactivated virus or subunits of the virus, such as the surface
Characteristics of live virus vaccines versus nonliving
proteins of the virus. The original Salk poliovirus vaccine
vaccines are compared in Table 10.3
and current vaccines against influenza and rabies viruses
Modern biotechnology makes possible another approach
use inactivated virus. For these vaccines, virus is prepared
to subunit vaccines, the use of a nonpathogenic virus as
and purified, and virus infectivity is destroyed by treatment
a vector to express proteins from a virulent virus. This
with formalin or other inactivating agents. These inactivated
approach potentially overcomes many of the disadvantages
viruses are injected, often intramuscularly, and induce an
of inactivated virus vaccines, or of subunit vaccines based on
immune response. Subunit vaccines, on the other hand, are
purified components, because the antigens are presented in
usually produced by expressing the surface proteins of a
the context of a viral infection and in a native conformation.
virus in a cell culture system. The proteins are purified and
To date, none of the licensed vaccines use this procedure, but
injected. Licensed subunit vaccines include the modern vac-
clinical trials are in progress or are planned to begin soon for
cine against hepatitis B virus.
a number of such vaccines. Two examples will be cited. In
Inactivated virus vaccines or subunit vaccines suffer from
one series of trials, vaccinia virus is being used to express the
a different set of problems from live virus vaccines, but in
HIV surface glycoproteins. Antibodies to the HIV glycopro-
turn have a number of advantages. Their advantages include
teins are induced, but whether they are protective remains to
the fact that they are usually stable; that interference by
be determined. In another series of trials, the vaccine strain
infection with other viruses does not occur; and, with proper
of yellow fever virus has been engineered to express the sur-
monitoring, viral virulence is not a problem. Their stability
face glycoproteins of Japanese encephalitis virus, of dengue
makes transport and storage of the vaccines easier and more
virus, or of West Nile virus. Based on the successful use of
reliable. Their insensitivity to interference makes it possi-
yellow fever vaccine for more than 50 years, the prognosis is
ble to immunize against many viruses simultaneously. The
good that these new vaccines will be successful. The use of
fact that the viruses are inactivated, or that no live virus was
viruses as vectors is covered in more detail in Chapter 11.
ever present in a subunit vaccine, means that no virus infec-
tion occurs with its potential for disease. Early lots of the
Salk inactivated poliovirus vaccine were contaminated with
DNA Vaccines
live poliovirus, which resulted in a number of cases of polio
A new and potentially exciting approach to vaccination is
caused by the vaccine, but better methods of inactivation and
to inject DNA that encodes genes whose expression leads to
of monitoring residual infectivity have solved this problem.
immunity to a virus. This approach has been tested in model
Difficulties with these vaccines include the expense of pre-
systems with promising results and clinical trials in humans
paring the large amounts of material required, the necessity for
have recently begun. Plasmid DNA containing the gene for,
multiple inoculations in the case of most such vaccines, and the
failure to induce a full range of immune responses. Because
no virus replication occurs, large amounts of material must be
TABLE 10.3 Characteristics of Live and
injected to induce an adquate immune response. Further, lack
Killed Vaccines
of virus replication means that an inflammatory response that
is required for an efficient immune response must be obtained
Live attenuated
Killed virus
by using adjuvents that are incorporated into the vaccine. In
Activity
virus vaccine
and subunit
practice, inactivated virus or subunit vaccines are designed to
Antibody induction
+++
+++
provoke only a very limited inflammatory response, and mul-
MHC class I (CD8 cells)
+++
tiple immunizations are usually required in order to achieve
Cytotoxic T-cells
+++
effective immunity. Even so, a full range of immunity is not
MHC class II (CD4 cells)
+++
+++
achieved. Worse, in two cases immunization with inactivated
virus resulted in more serious illness on subsequent infection
Humoral antibody
+++
+++
with epidemic virus. Inactivated virus vaccines against mea-
All viral antigens
Usually
Seldom
sles virus and respiratory syncytial virus did not protect against
Longevity of immunity
Months/years
Months
infection by the respective viruses, and led to the development
Cross-reactivity among viral
+++
+
of atypical disease that was more severe than that caused by
strains
the viruses in nonimmune people. The measles vaccine was
Risk of viral disease
+
subsequently replaced with a live virus vaccine, which has
been very successful, but no successful vaccine has yet been
Source: Data for this table came from Granoff and Webster (1999),
developed for respiratory syncytial virus.
p. 1862 and from Fields et al. (1996), p. 371.
say, a surface glycoprotein of influenza virus under the con-
transmissible gastroenteritis virus (TGEV) were better pro-
trol of a general mammalian promoter is injected intramus-
tected from TGEV infection than were animals vaccinated
cularly. Muscle cells take up DNA and import it into the
with a commercial live TGEV vaccine. Mice immunized
nucleus, a surprising finding. Expression of the influenza
with a single dose of DNA expressing the measles H protein
gene may elicit an immune reaction against the encoded
and then multiply boosted by being fed transgenic tobacco
influenza protein. Immunization with DNA might produce
plants expressing measles H protein developed significantly
protective immunity in the best of cases, or DNA immuni-
higher neutralizing antibody titers to measles virus than did
zation might serve to prime an individual for later inocula-
mice immunized with either the DNA or the plant food alone.
tion with a conventional vaccine so as to obtain a stronger
Thus, in principle plant-based vaccines are capable of induc-
response.
ing immunity. Whether practical vaccines can be developed
Immunization with DNA has many potential advantages.
using this approach remains a question for the future.
The encoded protein is made intracellularly and therefore a
CTL response is generated, because peptides are presented
Vaccine Development: The Example of
by class I MHC molecules. In addition, the proteins are made
Measles Vaccines
and are present in a native conformation, which may make
possible the production of antibodies against a wider range
Vaccines against viruses have been enormously success-
of conformational epitopes. Other advantages include the
ful in controlling viral diseases that have plagued mankind
fact that multiple proteins can be encoded in plasmid DNA,
for millennia. Smallpox virus has been eradicated world-
which allows immunization against multiple products of one
wide, poliovirus has been eradicated from the Americas and
virus or against several different viruses, and that cytokine
may be eradicated worldwide within the next few years, and
genes can also be present in the plasmid, which can serve
many epidemic diseases have been controlled, at least in
as adjuvents to obtain not only a stronger immune response
regions that have access to the vaccines. As an example of
but to direct the immune response toward desired pathways.
potential difficulties in vaccine development, however, the
Another important feature is that the expression of the for-
measles virus vaccines will be discussed. Measles virus is
eign protein continues for some time, potentially leading to
ubiquitous. Not so long ago, virtually the entire population
a better immune response. Finally, plasmid DNA is easy to
of the world was infected by the virus as children. Very few
modify to express different genes, cheap to make in quan-
people escaped infection by the virus. Measles can be a seri-
tity, and easy to purify. Once safety trials have been con-
ous illness, with significant mortality and debilitating seque-
ducted for a DNA vaccine, it should be possible to modify it
lae (Chapter 4). Concerted efforts to produce a vaccine began
to express antigens from other viruses with minimal safety
many years ago. One early measles virus vaccine, used from
concerns.
1963 to 1967, was made with inactivated virus. Vaccination
Human trials with DNA vaccines are just beginning.
with this vaccine potentiated a more serious illness when a
A Phase 1 safety trial of an HIV vaccine has just been com-
person was infected with the epidemic virus, however, char-
pleted using 86 volunteers, and the results were sufficiently
acterized by a more severe rash and called "atypical mea-
encouraging to begin Phase 2 efficacy trials. The DNA vac-
sles." The atypical disease is thought to be produced by an
cine uses multiple DNAs expressing HIV-1 genes from
atypical or unbalanced T-cell response to the virus following
three subtypes of the virus. Three intramuscular injections to
immunization. This response controls virus infection only
prime the individuals were given using a needle-free device.
poorly and leads to collateral damage from T-cell attacks on
This was followed by a boost using adenovirus type 5 that
virus-infected cells. An attenuated virus vaccine was more
expressed HIV proteins. At the highest doses used, almost
successful. The first live virus vaccine used was protective
all individuals produced antibodies against HIV as well as
but insufficiently attenuated and thus more reactogenic than
both CD4+ and CD8+ T cells that were reactive against HIV
would be ideal. It was subsequently replaced with a more
antigens.
highly attenuated vaccine.
The attenuated virus vaccine currently in use has been
highly successful in controlling measles in many develop-
Plant-Based Vaccines
ing countries as well as in the developed countries. The dis-
Another possible approach to the production of vaccines
tribution of the virus as of 1998 is shown in Fig. 10.13A,
is to use modern biotechnology to introduce genes for viral
illustrating that by this time the virus had been eradicated in
proteins into a food plant. If eating this plant induces immu-
many developed countries and had substantially reduced the
nity, it would provide a cheap and easy alternative to vaccines
incidence of measles over most of the world. By 2004, the
that require extensive purification followed by injection. No
virus had been essentially eradicated from the Americas and
human vaccines have been developed to date using this tech-
most other areas, but pockets of endemicity remain in Africa
nology, but the concept has been tested in animals. Piglets
(Fig. 10.13B) where the virus continues to be an impor-
fed transgenic corn that expresses the spike protein of swine
tant cause of infant mortality and morbidity. The failure
A
1998
Equator
Cases of Measles
per 100,000 Population
>100
10-100
1-10
None
No data
B
2004
Moroc-
co
Niger
Cases of Measles
per 100,000 Population
1.6-5.0
0.5-1.5
0.1-0.5
<0.1
No data
FIGURE 10.13 Incidence of measles. (A) Reported incidence rate of measles per country as of 14 August 1998. Rates
are shown as cases per 100,000 population. From Web site of World Health Organization. (B) The incidence of measles
in Africa in 2004. Note the drastically different scales. In 2004 the Americas were virtually free of measles, although
Southeast Asia (including India) still records upwards of 200,000 deaths from measles annually.
Search WWH :