outside the cell and the N terminus inside, with a transmem-
In the [PSI] state, Sup35p assumes an altered conforma-
tion and aggregates, like PrPSc. The [PSI] state is dominant
brane domain near the middle of the molecule. Preliminary
data suggest that this form of PrP is neurotoxic: CtmPrP has
and can be transmitted to other yeast cells by transfer of
been found in brains of animals, including humans, suffer-
cytoplasm containing [PSI]. Thus, the prion state induces
ing from TSE but not in normal brains. This has led to a
the normal cell protein to assume the prion state, as with
model in which CtmPrP is regularly produced at some fre-
the model for PrPSc. The effect of the [PSI] state on the cell
quency, but the normal cell has a mechanism to eliminate it.
is to render Sup35p nonfunctional, and thus has the same
Overproduction of CtmPrP, either by mutation or by a failure
effect as deletion of the gene encoding the protein. Loss
to eliminate it, leads to the symptoms of TSE. In this model,
of Sup35p activity leads to increased readthrough of stop
production of PrPSc might somehow result in the accumula-
codons during translation, and renders nonsense suppres-
tion of CtmPrP, perhaps by overwhelming the ability of the
sor tRNAs much more active. [URE3] is the prion state
cell to eliminate it.
of Ure2p, a protein involved in nitrogen catabolism. Like
[PSI], [URE3] is an aggregated form of a conformational
variant of Ure2p, and is dominant and transmissible. Loss
PRIONS OF YEAST
of Ure2p by the cell affects the metabolism of nitrogen.
Normal cells can assume the prion state with a low fre-
Prions, defined as agents that possess two (or more)
quency, but once assumed the prion state is retained. Cells
conformational forms, a soluble "normal" form and an
in the prion state can be cured by certain treatments that
aggregated form that can induce the conversion of the nor-
break up the protein aggregates and cause the protein to
mal form to more of itself, have also been found in fungi.
assume a normal conformation. Studies of yeast prions
Two prions have been found in yeast (Saccharomyces
have shown that Sup35p produced in bacterial cells can be
cerevisiae) and a third in Podospora spp. The yeast prions
converted to [PSI] in vitro by introduction of a small seed
have the characteristics of disease but the Podospora prion
of [PSI]. The [PSI] produced in vitro can be used in turn to
performs a normal cell function (controlling heterokaryon
convert more Sup35p to [PSI]. The [PSI] produced in vitro
compatibility). The yeast prions are called [URE3], which
is infectious--when introduced into yeast it induces the
affects nitrogen catabolism, and [PSI], which affects the
assumption of the prion state. Thus, these studies clearly
termination of polypeptide chains during translation. A
show that yeast prions are infectious and that only protein
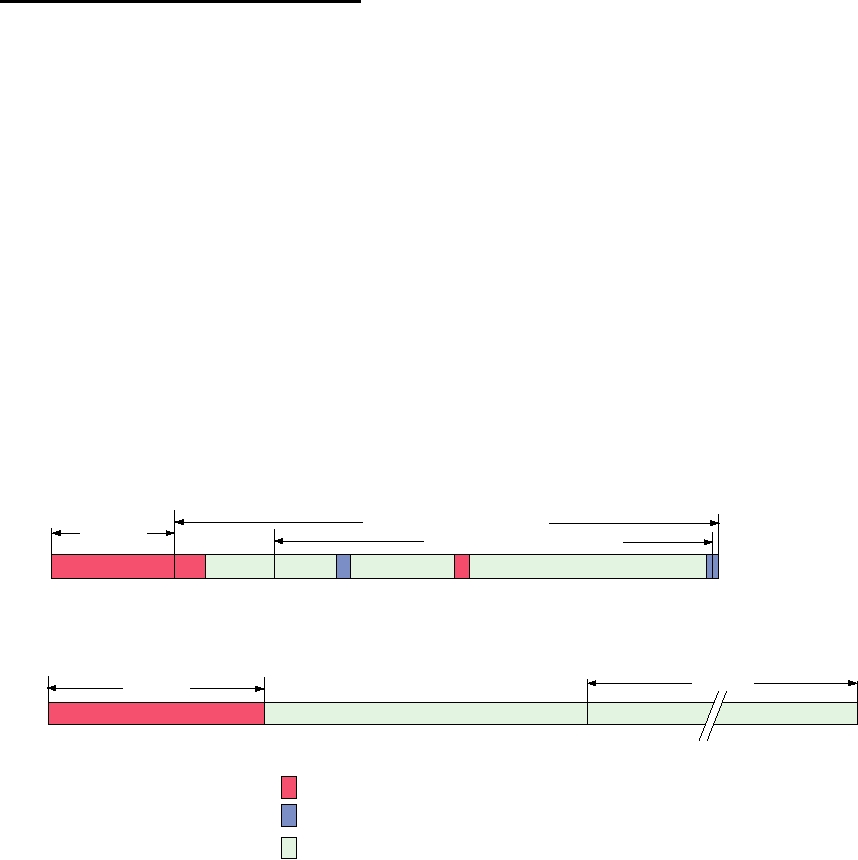
diagram of these proteins is shown in Fig. 9.17. [PSI] is a
is required for infectivity, providing further support for the
prion form of Sup35p, which is a translation release factor.
protein-only hypothesis of mammalian TSEs.
Yeast Prion Protein Ure2p
Prion-inducing
N-repression domain
domain
Glutathione-S-transferase
1
65 80
151 158
221 227
348 354
Yeast Prion Protein Sup35p
Translation-termination
Prion-propagating
domain
domain
1
114
254
685
Prion-promoting sequences
Prion-inhibiting sequences
Domains with known nonprion functions
FIGURE 9.17
Comparison of two yeast prion proteins. The prion domains (red) of Ure2p and Sup35p are rich
in Asn and Gln residues, which are important for prion generation and propagation. Adapted from Figure 10 of Wickner
et al. (1999).
Search WWH :