have resulted from an abundance of pine nuts in the area dur-
SIVrcm (red-capped mangabey)
ing a good growing year, leading the local people to harvest
LTR
LTR
larger amounts of these than usual and store them in their
gag
env
vif
homes when their normal storage areas became full. With
pol
abundant food available, the rodent population exploded and
vpr
nef
tat
invaded homes to get to the pine nuts, and it is thought that
rev
this more intimate contact between humans and rodents may
have led to the epidemic. The hantavirus responsible for this
vpu
epidemic is now called Sin Nombre virus, which is Spanish
for "without a name." Early suggestions that it be called Four
SIVcpz (chimpanzee/HIV-1)
Corners virus or Muerto Canyon virus (after a geographical
LTR
LTR
feature in the area) drew objections from local residents who
gag
env
vif
did not want this major tourist area identified with a fatal
pol
disease. Eventually the CDC simply named it Sin Nombre
vpr
nef
tat
(there is a small creek in the area called the Sin Nombre
rev
River that serves as justification for the choice of name).
With the discovery of Sin Nombre virus, searches for
vpu
viruses in other regions of North America resulted in the iso-
SIVgsn/SIVmus/SIVmon
lation of many viruses related to Sin Nombre. These viruses
LTR
LTR
are associated with other rodents in the order Sigmodontinae
and have been given names of local features in order to
gag
env
vif
distinguish them. These include New York, Monongahela,
pol
vpr
nef
Bayou, and Black Creek Canal viruses, all of which have
tat
caused HPS in the United States (see Fig. 4.26). Related
rev
viruses are also found in Latin America. In fact, studies have
vpu
now shown that hantaviruses are present in virtually all states
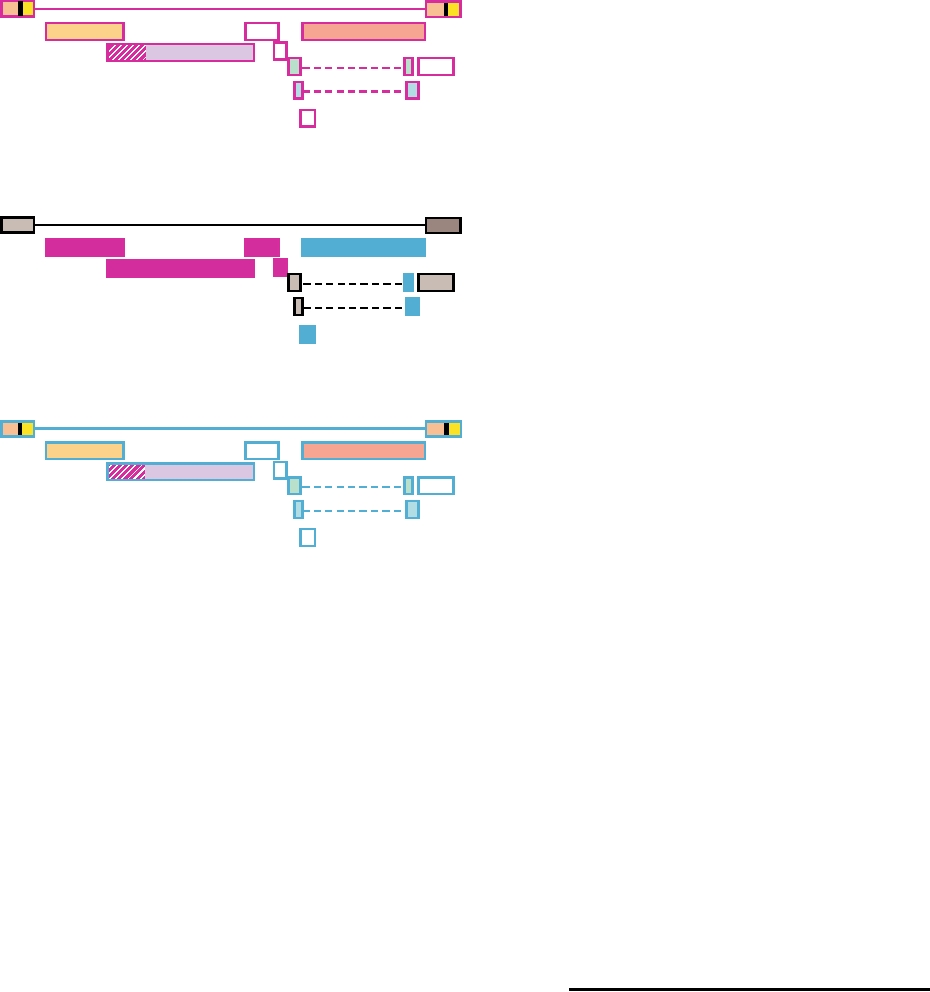
FIGURE 8.13 Diagram of the recombinant origin of SIVcpz. The various
within the United States and into Latin America, and that
genes of SIV in red-capped mangabeys are shown at the top, outlined in
fatalities due to infection by the virus have occurred in most
magenta. Similarly the genome of SIVgsn (greater spot-nosed)/SIVmus
states. Retrospective studies of stored sera collected from
(mustached)/SIV mon (mona) monkeys is shown at the bottom, outlined
patients who died of ARDS in the past have identified earlier
in blue. In the recombinant genome of chimpanzee/HIV-1 (center), SIVcpz
cases of HPS. Thus these viruses are widespread and have
genes derived from SIVrcm are magenta, genes from SIVgsn are in blue,
and genes of unknown origin are in gray. Adapted from Figure 4 in Sharp
caused many fatal cases of human disease over the years.
et al. (2005).
As noted in Chapter 4, the epidemiology of arenavi-
ruses is similar to that of the hantaviruses. Several South
American arenaviruses have caused increasing numbers
of cases of human hemorrhagic fever because of increased
excreta. The first hantavirus to come to medical attention
contact between humans and the rodent carriers of the
was Hantaan virus which caused more than 3000 cases of
viruses. The development of the Pampas of Argentina, in
hemorrhagic fever with renal syndrome in U.S. troops dur-
particular, led to increased incidence of human arenavirus
ing the Korean War. Since then, many hantaviruses have been
disease.
identified in both the Old World and in the Americas that
cause serious human illness. They are examples of emerg-
ing viruses because as the number of humans increases and
FUR THER READING
as they invade more habitat occupied by rodents carrying
hantaviruses, the incidence of infection in humans has risen.
Emerging Viral Zoonoses
Very interesting in this regard was the isolation, in May 1993,
of a new hantavirus that causes acute respiratory distress in
Anishchenko, M., Bowen, R. A., Paessler, S., et al. (2006). Venezuelan
humans that can lead to rapid death, a syndrome now called
encephalitis emergence mediated by a phylogenetically predicted viral
hantavirus pulmonary syndrome (HPS) and originally called
mutation. Proc. Natl. Acad. Sci. U.S.A. 103: 49944999.
acute respiratory disease syndrome (ARDS). The virus
Barclay, W. (2006). Influenza vaccines. Microbiol. Today 33(1): 1719.
Davis, C. T., Ebel, G. D., Lanciotti, R. S., et al. (2005). Phylogenetic analy-
was isolated by the CDC in collaboration with local health
sis of North American West Nile virus isolates, 20012004: evidence
authorities following an epidemic in the Four Corners area
for the emergence of a dominant genotype. Virology 342: 252265.
of the southwestern United States that resulted in approxi-
Eaton, B. T., Broder, C. C., Middleton, D., and Wang, L.-F. (2006). Hendra
mately 25 deaths. The virus is associated with the deer mouse
and Nipah viruses: different and dangerous. Nature Rev. Microbiol. 4:
Peromyscus maniculatus. It is thought that the epidemic may
2335.
Mackenzie, J. S., Field, H. E., and Guyatt, K. J. (2003). Managing emerg-
Johnson, R. T. (2003). Emerging viral infections of the nervous system.
ing diseases borne by fruit bats (flying foxes), with particular refer-
J. Neurovirol. 9: 140147.
ence to henipaviruses and Australian bat lyssavirus. J. Appl. Microbiol.
Kallio-Kokko, H., Uzcategui, N., Vapalahti, I., and Vaheri, A. (2005). Viral
94: 5969S.
zoonoses in Europe. FEMS Microbiol. Rev. 29: 10511077.
Mayen, F. (2003). Haematophagous bats in Brazil, their role in rabies
Kobasa, D., and Kawaoka, Y. (2005). Emerging influenza viruses: past and
transmission, impact on public health, livestock industry and alterna-
present. Curr. Mol. Med. 5: 791803.
tives to an indiscriminate reduction of bat population. J. Vet. Med. 50:
Olsen, B., Munster, V. J., Wallensten, A., et al. (2006). The global patterns
469472.
of influenza A virus in wild birds. Science 312: 384388.
Osborne, J. C., Rupprecht, C. E., Olson, J. G., et al. (2003). Isolation of
Peters, C. J. (2006). Emerging viral diseases. Chapter 18 in: Fields
Kaeng Khoi virus from dead Chaerephon plicata bats in Cambodia.
Virology, Fifth Edition (D. M. Knipe and P. M. Howley, Eds. in chief),
J. Gen. Virol. 84: 26852689.
Philadelphia, Lippincott Williams & Wilkins, pp. 605626.
Reynes, J.-M., Counor, D., Ong, S., et al. (2005). Nipah virus in Lyle's fly-
Sharp, P. M., Shaw, G. M., and Hahn, B. H. (2005). Simian immunodefi-
ing foxes, Cambodia. Emerg. Infect. Dis. 12: 10411047.
ciency virus infection of chimpanzees. J. Virol. 79: 38923902.
Warrilow, D. (2005). Australian bat lyssavirus: a recently discovered new
Siquiera, J. B., Jr., Martelli, C. M. T., Coelho, G. E., da Rocha Simplício,
rhabdovirus. Curr. Top. Microbiol. Immunol. 292: 2544.
A. C., and Hatch, D. L. (2005). Dengue and dengue hemorrhagic fever,
Woo, P. C. Y., Lau, S. K. P., Li, K. S. M., et al. (2006). Molecular diversity
Brazil, 19812002. Emerg. Infect. Dis. 11: 4853.
of coronaviruses in bats. Virology 351: 180187.
Wang, E., Ni, H., Xu, R., et al. (2000). Evolutionary relationships of endemic/
epidemic and sylvatic dengue viruses. J. Virol. 74: 32273234.
Webster, R. G., Peiris, M., Chen, H. L., and Guan, Y. (2006). H5N1 out-
SARS
breaks and enzootic influenza. Emerg. Infect. Dis. 12: 38.
Vijayanand, P., Wilkins, E., and Woodhead, M. (2004). Severe acute respi-
ratory syndrome (SARS): a review. Clin. Med. 4: 152160.
Bats and Viruses
Stadler, K., Masignani, V., Eickmann, M., et al. (2003). SARS--Beginning
Dobson, A. P. (2005). What links bats to emerging infectious diseases?
to understand a new virus. Nature Rev. Microbiol. 1: 209218.
Science 310: 628629.
Poon, L. L. M., Chu, D. K. W., Chan, K. H., et al. (2005). Identification of
Fooks, A. R., Brookes, S. M., Johnson, N., McElhinney, L. M., and Huston,
a novel coronavirus in bats. J. Virol. 79: 20012009.
A. M. (2003). European bat lyssaviruses: an emerging zoonosis.
Li, W., Shi, Z., Yu, M., et al. (2005). Bats are natural reservoirs of SARS-
Epidemiol. Infect. 131: 10291039.
like coronaviruses. Science 310: 676679.
Jia, G., Zhang, Y., Wu, T., Zhang, S., and Wang, Y. (2003). Fruit bats as a
Snijder, E. J., Bredenbeek, P. J., Dobbe, J. C., et al. (2003). Unique and
natural reservoir of zoonotic viruses. Chin. Sci. Bull. 48: 11791182.
conserved features of genome and proteome of SARS-coronavirus, an
Leroy, E. M., Kumulungui, B., Pourrut, X., et al. (2005). Fruit bats as reser-
early split-off from the coronavirus group 2 lineage. J. Mol. Biol. 331:
voirs of Ebola virus. Nature 438: 575576.
9911004.
Search WWH :