Emerging and Reemerging Viral Diseases
The litany of the viruses described in the previous chap-
HIV, became human viruses while others, like influenza
ters of this volume makes clear that humans have been sub-
virus, remain zoonotic viruses. There is growing concern
jected to a large number of viral diseases throughout our
that viruses to date have caused small epidemics in humans
history. Some of these viruses evolved along with humans
but may acquire the ability to cause very large epidemics.
and have been present since the earliest human walked the
In this chapter we will consider a number of viruses that are
earth. Such viruses include the various herpesviruses, for
known to have caused epidemics in humans only within the
example, which were present as human pathogens at the
last century, or that have the potential to cause wide-rang-
time that humans first appeared. Others have been acquired
ing epidemics in the future, or that are undergoing dramatic
from zoonotic sources. These are animal viruses that have
range expansion at present.
acquired the ability to infect humans. Upon jumping from
their animal sources to humans, some of these viruses
BAT-ASSOCIATED VIRUSES
became human viruses that infect only humans, and humans
became the vertebrate reservoir of this new virus. Such
viruses include measles, described in Chapter 4, and the
A number of emerging viruses are bat viruses that have
dengue viruses, described in Chapter 3 and in this chapter.
recently entered the human population and caused small or
Many of these viruses entered the human population long
large epidemics of disease. Although these viruses can cause
ago. Arguments were presented in Chapter 4 that measles
serious illnesses in humans, they usually cause little or no
virus could not have existed as a human virus until perhaps
illness in bats. The recent emergence of bat viruses as human
5000 years ago when the human population first reached the
pathogens may seem strange because so many viruses are
numbers required to sustain the virus in the population, and
now known to come from bats, but in fact bats form a siz-
that this virus probably jumped from cattle to humans after
able proportion of the diversity of mammals. More than 900
humans domesticated these animals. Others have entered the
species are currently recognized and these constitute more
human population more recently. The four serotypes of den-
than 20% of all mammalian species. Furthermore, bats are
gue virus, for example, appeared to have jumped indepen-
intensely social creatures that are ideally suited to pass
dently from monkeys to humans between 200 and 1000 years
viruses back and forth among large populations. Humans
ago, and HIV was established as a human virus within the
impinge more and more into the habitats of bats, and this,
last 50100 years. Other zoonotic viruses that infect humans
as well as disruptions of bat colonies caused by humans,
do so only peripherally and humans do not serve as the ver-
has led to more contact between bats and humans or their
tebrate reservoir of these viruses. Examples are West Nile
domestic animals. Furthermore, in many areas of the world
virus, Eastern equine encephalitis virus, Ebola virus, and
bats are used as food or for medicinal purposes, resulting in
rabies virus.
humanbat contacts.
As the human population expands it impinges on wildlife
Almost all bats are nocturnal. They are classified in the
more and more, and changes in habitat caused by humans
order Chiroptera, which has two major divisions or sub-
lead to closer interactions between humans and wildlife, with
orders. Megachiroptera are mostly large, fruit-eating bats
the result that an increasing number of zoonotic viruses are
that are classified in a single family, Pteropodidae. There
causing epidemics of serious human disease. Some, like
are about 170 species distributed throughout the tropics of
the Old World. They find their food, consisting of fruits,
of human cases of rabies have been bat-associated rabies. In
flowers, and pollen, using eyesight and an excellent sense
some of these cases of bat rabies in humans, exposure to bats
of smell. Of these, more than a third, 65, belong to the genus
is documented and the bite of infected bats is known to have
Pteropus and are called flying foxes. The Pteropus flying
transmitted the virus, but in other cases there is no known
foxes are found from Australia across southern Asia and
contact with bats, leading to the suggestion that inhaling
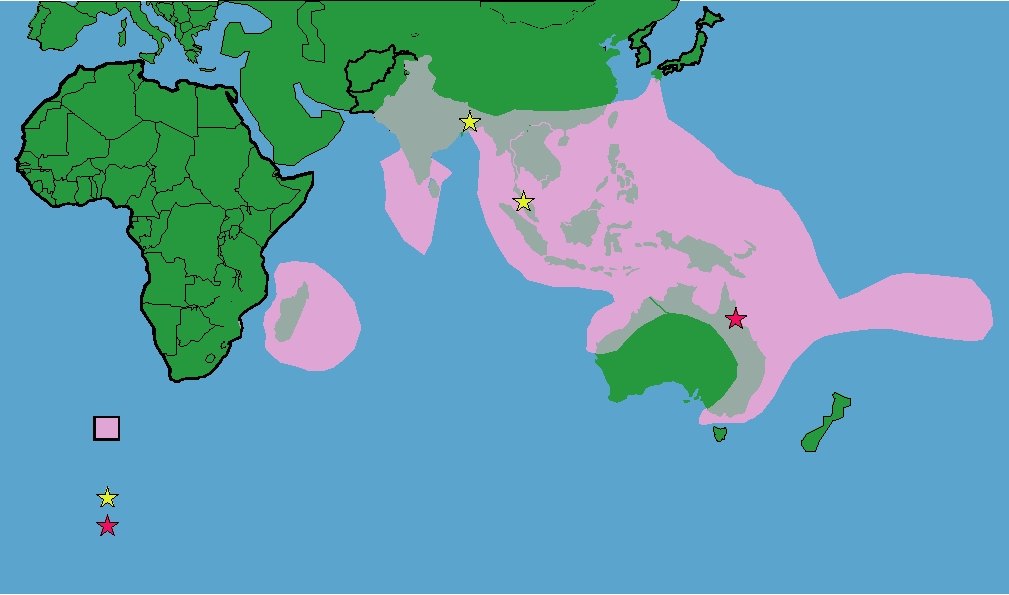
India to Madagascar (Fig. 8.1). They weigh from 300 grams
aspirated droplets containing rabies may be the cause of the
to more than a kilogram and have a wingspan of 0.6 to 1.7
infection.
meters. Microchiroptera are in general smaller and most eat
In South America, vampire bats, which feed on the blood
insects. They are virtually worldwide in distribution. They
of mammals after biting them with their sharp incisors, have
have evolved echolocation to navigate and find their prey in
been an important vector in the spread of rabies to livestock
the dark. Bats play an important role in the ecology of the
and humans. This has resulted in campaigns to indiscrimi-
planet, dispersing seeds, pollinating plants, and reducing the
nately slaughter bats using a variety of methods, including
number of night flying insects such as mosquitoes.
poison and the destruction of roosts and caves with explo-
sives. Although these campaigns have resulted in enormous
numbers of bats being killed, these campaigns have had no
Rabies Virus
effect on the spread of rabies. Thus, reduction of bat num-
bers is not effective in the control of rabies but does destroy
Rabies virus is an example of a virus for which bats are
ecologically important animals.
an important reservoir. Although we think of rabies as being
The various bat lyssaviruses, which can also cause rabies
primarily associated with canines such as dogs and other
in humans, were described in Chapter 4. Although only a few
mammals such as skunks and raccoons, and these animals
human cases are known that arose from infection by these
do serve as important reservoirs for rabies that enter the
viruses, they have the potential to spread more widely. Further,
human population, bats are also an important reservoir. In
bites or scratches from bats need to be treated as potential
fact, in the United States over the last few years the majority
Range of the genus Pteropus (Flying Foxes)
Outbreaks
Nipah virus
Hendra virus
FIGURE 8.1 Illustration of the distribution of the genus Pteropus in the Old World. These large bats, called flying
foxes, are present from Madagascar across the Indian subcontinent and throughout the tropical and subtropical regions
of Indonesia, Australia, and the Philippines, and as far east as the Cook Islands. Adapted from Figure 1B in Eaton et al.
(2006).
exposure to rabies and treated accordingly. This is expensive
A related virus, 83% identical to Hendra virus at the
and in effect only available in developed countries.
amino acid level, emerged in 1998 that represents a much
more serious threat to human health. From September 1998
to April 1999, an outbreak of 258 cases of human encepha-
Henipaviruses
litis occurred in Malaysia and Singapore that had a 40%
In September of 1994, a number of cases of severe respira-
mortality rate. Clinical symptoms included fever, headache,
tory illness occurred in racehorses near Brisbane, Australia.
myalgia (muscle aches), drowsiness, and disorientation that
The first horse to become ill was a pregnant mare that was
sometimes progressed to coma within 48 hours. The disease
pastured in a field, which was then moved into a stable with 23
was associated with an outbreak of respiratory disease in
other thoroughbreds. The disease spread among the horses in
pigs with or without neurological symptoms, and humans
this stable and to an adjoining stable and ultimately 17 horses
infected with the disease were pig farmers or others closely
became ill, of which 13 died. Of the four horses that survived,
associated with pig farming. It was first thought that the
two were left with mild neurological sequelae. Three other
outbreak was due to infection by Japanese encephalitis (JE)
horses were infected but did not suffer symptoms. Two humans
virus (see Chapter 3 on the importance of pigs as amplify-
that nursed the horses became ill with a severe respiratory
ing hosts for this virus) and the Malaysian government vac-
disease, of whom one died of respiratory and kidney failure.
cinated 2.4 million pigs against JE virus. When this did
Spread of the disease required close contact, and imposition
not slow the epidemic, 1.1 million pigs were culled in an
of quarantine measures contained the outbreak. A previously
attempt to reduce the incidence of disease. In March 1999,
unknown virus was isolated from the sick animals that was
with the assistance of the Centers for Disease Control and
found to be a paramyxovirus and it was initially named equine
Prevention, the virus responsible for the epidemic was iden-
morbillivirus. Sequencing of the genome showed that it was
tified as a Hendra-like virus, a virus related to but distinct
not closely related to the morbilliviruses; however, and the
from Hendra virus. Retrospective studies suggested that the
virus was assigned to a new genus called Megamyxovirus
virus had been responsible for disease in pigs in Malaysia for
because of the large size of the genome. Subsequent stud-
several years and it seems clear that the human cases were
ies established that the virus was a bat virus and widespread
contracted from pigs. There is no evidence for human-to-
in flying foxes in eastern Australia. The virus was renamed
human transmission in this outbreak. The virus responsible
Hendra virus after the Brisbane suburb where the outbreak
has been called Nipah virus, after the village in Malaysia
occurred and the genus was renamed Henipavirus. Analysis
where the disease first appeared, and it is classified as a sec-
of sera from healthy humans and horses in the area failed to
ond member of the genus Henipavirus (which gets its name
detect the presence of antibody, and analysis of more than
from Hendra and Nipah viruses).
5000 sera from a variety of wild animals trapped in the areas
Like Hendra virus, the reservoir of Nipah virus is flying
also failed to detect antibody in any animal other than fly-
foxes and the virus has been isolated from flying foxes in the
ing foxes. Flying foxes can be readily infected experimentally
area. It has been suggested that the outbreak occurred in part
with the virus but do not suffer illness upon infection.
because the destruction of the natural habitat of the flying
A second outbreak of Hendra virus began in August 1994
foxes caused by deforestation and consequent food shortage
about 1000 km north of Brisbane. Two horses died and the
led the bats to forage in nearby orchards located very near
owner of the horses became mildly ill with neurological
piggeries. There, half-eaten fruit or regurgitated fruit that
symptoms from which he appeared to recover. However,
was contaminated with virus-containing saliva from the bats
in October of 1995 the owner suffered a relapse and died
could be eaten by pigs, causing them to become infected.
of encephalitis. At this point an investigation showed that
More recent epidemics of Nipah virus encephalitis
Hendra virus was to blame for the illness of the horses and
have occurred in southern Asia. Epidemics in Bangladesh
the death of the owner. In January 1999, a fatal case of
occurred in 2001, 2003, 2004, and 2005. No evidence for
Hendra infection occurred in a horse near Cairns, Australia,
the intermediate infection of an animal, as occurred in the
and in 2004 a horse died of Hendra infection in Townsville.
Malaysian epidemic, has been seen in these epidemics.
In the 2004 incident the veterinarian who attended the horse
Furthermore, in the 2004 epidemic evidence was obtained
was infected but recovered after a mild illness.
that person-to-person transmission of the virus had occurred.
Extensive studies of flying foxes have shown that Hendra
It is likely that the disease was transmitted directly from bats
virus is present in all four species of flying fox that occur in
to humans, possibly by human consumption of partially
Australia. Almost half of flying foxes have been found to
eaten fruit that was contaminated with bat saliva contain-
have antibodies to the virus, so it is widespread and com-
ing the virus, followed by person-to-person transmission.
mon. It is only rarely transmitted to other animals, however,
It is known in Bangladesh, for example, that during the
at least to date, as shown by the extensive serological studies
fruiting season young boys climb trees to pick fruit. If this
and the limited occurrence of clinical illness caused by the
fruit was partially eaten by bats, the fruit could be contami-
virus in humans and their horses.
nated with the virus from the bat. The fatality rate in these
epidemics was as high as 75%. In nearby India, an epidemic
have been infected by the virus. In the autumn of 2002 an
of Nipah occurred in 2001. Flying foxes are widely distrib-
epidemic of SARS in humans began in Guangdong Province
uted throughout this area (Fig. 8.1) and Nipah virus has been
in China. The disease is an atypical pneumonia characterized
isolated from them in both Malaysia and Bangladash. The
by high fever, myalgia, and lymphopenia (smaller numbers of
virus has also been isolated from flying foxes in Cambodia
lymphocytes). By February of 2003 there were 305 cases with
although human infection has not been documented to date.
five deaths. The infection was then spread to other areas by a
The very wide distribution of Hendra and Nipah viruses, the
Chinese doctor who had been treating patients in Guangdong.
possibility of person-to-person transmission, and the increas-
He traveled to Hong Kong on 21 February 2003 and while
ing contacts between humans and their domestic animals with
staying in a hotel he developed symptoms of SARS and died
fruit bats carrying the virus, suggests that epidemics will con-
shortly thereafter. Ten guests at the hotel who were housed
tinue to occur. As indicated for rabies, eradication of the bats
on the same floor or nearby floors became infected and
is neither desirable nor feasible. However, simple solutions
before developing symptoms traveled to Singapore, Vietnam,
exist to reduce the contacts of humans and their animals with
Canada, and the United States, spreading the epidemic. The
the bats, such as not locating fruit orchards near piggeries.
epidemic also spread independently to Beijing in April of
2003. The epidemic finally waned in the summer of 2003
when the World Health Organization reported the cumulative
SARS Coronavirus
total of 8098 probable cases of SARS with 774 deaths world-
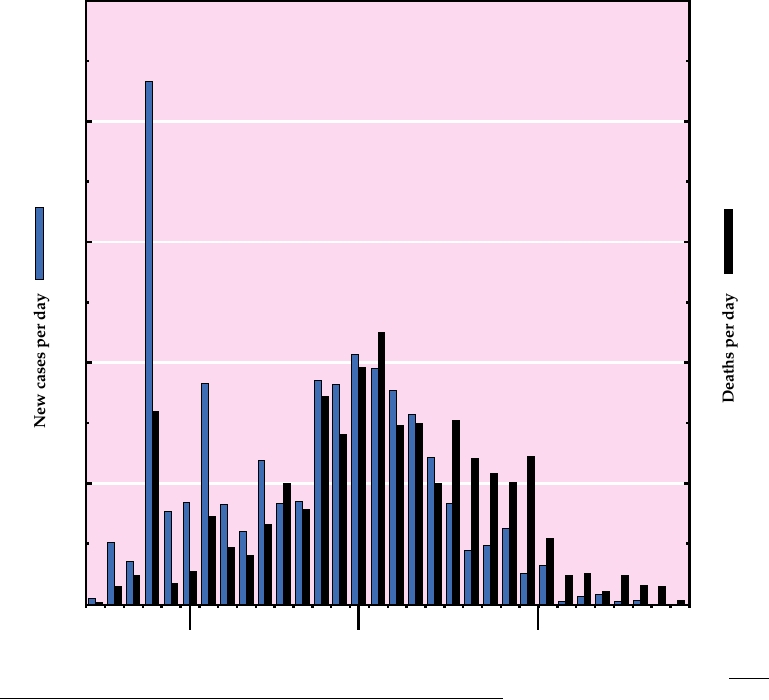
wide in 29 countries (Fig. 8.2). The death rate from the disease
SARS virus (severe acute respiratory syndrome virus)
was thus about 10% although it is age related. Children either
occurs in a number of cave-dwelling species of horseshoe
do not contract the virus or show little reaction to it, whereas
bats in China belonging to the genus Rhinolophus. Field stud-
the death rate in people over 65 can be as high as 50%.
ies have found that 3070% of bats belonging to this genus
500
*
400
30
300
20
200
100
10
0
April
May
June
March
FIGURE 8.2
SARS epidemic of 2003. Cases and deaths reported between March 17 and June 23 are plotted. The data
for this graph were reported in the outbreak updates from the World Health Organization, and can be found at: http://
www.who.int/csr/don/archive/disease/severe_acute_respiratory_syndrome/en/. Cases and deaths have been normalized
for the length of the reporting interval, which varied from 2 to 4 days. The asterisk marks the date on which cumulative
totals were first released by China.
The disease probably started in markets in China in which
(also called CD13) by the human coronavirus 229E, trans-
a number of exotic animals including bats, masked palm civ-
missible gastroenteritus virus of swine, and feline infectious
ets (Paguma larvata), and raccoon dogs (Nyctereuctes pro-
peritonitis virus, and carcinoembryonic antigens by mouse
cyonoides) are sold for food. Civets and raccoon dogs from
hepatitis virus.
the markets were found to be infected by the virus and it is
believed that either these animals or bats being consumed
A Second Bat Coronavirus
as food spread the disease to humans, followed by human-
It is noteworthy that at least one other coronavirus,
to-human spread of the virus. It is almost certain that the
as yet unnamed, circulates in bats belonging to the genus
civets in the markets contracted the virus there from bats
Miniopterus. In Miniopterus pusillus more than 60% of the
because civets on farms were largely free from the SARS
bats were found to be positive for this virus. This virus is dis-
virus. In addition, 13% of tested merchants in the markets in
tinct from the SARS virus. It belongs to group 1 coronavi-
Guangdong had SARS antibodies (showing they had been
ruses whereas SARS belongs to group 2 coronaviruses. This
infected by the virus).
new virus is not known to infect humans or to cause disease.
Adaptation of SARS Virus to a Human Receptor
The Zoonotic Origin of a Human Coronavirus
Recent studies have shown that the SARS virus, a corona-
SARS is a zoonotic disease of humans caused by a coro-
virus, had to adapt to human receptors in order to cause severe
navirus. It is of interest that human coronavirus HCoV OC43
illness. Infection by the bat virus or the civet virus appears
also appears to have a zoonotic source. It is very similar to a
to cause only mild illness. As stated before, merchants who
virus of cattle, bovine coronavirus (BCoV). From studies of
were infected did not develop illness, and some persons who
the rate that mutations have been fixed in these viruses, it has
work with wildlife were found to be seropositive for SARS
been estimated that the virus entered the human population
but suffered no illness. However, several changes are present
around 1890.
in the virulent SARS virus isolated from humans. There is a
deletion of 29 nucleotides upstream of the start codon for the
Filoviruses
N protein and there are four amino acid changes in the spike
protein. It is believed that the crucial changes are two amino
Marburg Virus
acid changes in the spike protein that allow the virus to bind
The filoviruses first came to the attention of science
to the human receptor called ACE2 (angiotensin-converting
in 1967 when outbreaks of hemorrhagic fever occurred
enzyme 2) 1000-fold more avidly than does the civet strain
in Marburg and Frankfurt, Germany, and in Belgrade,
or the bat strain. This is perhaps the reason why the virus has
Yugoslavia. The cause was a virus subsequently named
not to date reappeared in the human population, together with
Marburg that was present in African green monkeys
extensive culling of animals in the food markets in China.
imported from Uganda whose kidneys were being processed
If occasional human cases occur they are likely to be mild
for cell culture production (for use in preparing poliovirus
unless the virus has the opportunity to mutate in humans to
vaccine). Twenty-five laboratory workers were infected and
form the virulent strain of the virus. However, this did hap-
six secondary cases resulted; of these 31 infected people,
pen in 2002 and may happen again in the future. There is
7 died. The monkeys in the shipment, which originated in
need to develop vaccines or antiviral treatments for the virus,
Uganda, also died. Subsequent studies with the virus iso-
as well as to maintain the Chinese food markets in a way that
lated during the outbreak showed that it caused lethal illness
does not encourage the spread of the virus.
in African green monkeys following experimental infection.
The ACE2 protein is highly conserved among mammals
There were 3 cases of Marburg in South Africa in 1975 (the
and it is perhaps surprising that one of the few amino acid
source of infection was probably Zimbabwe) with one death,
differences in the human form of this protein occurs in the
2 cases in Kenya in 1980 (infection probably in Uganda), 1
virus-binding site and causes such a change in the ability
case in Kenya in 1987, an outbreak of 149 cases with 123
of SARS to utilize ACE2 as a receptor. In view of the fact
deaths in Zaire (now the Democratic Republic of Congo) in
that to become virulent the virus must mutate to bind more
19982000, and an outbreak of 374 cases with 329 deaths
strongly to the human form of the ACE2 protein, it is inter-
in northern Angola in 2005. The number of cases is surely
esting that there is a second receptor for SARS virus, the
underreported since many people in remote areas do not seek
protein called CD209L or L-SIGN. Why this second recep-
medical assistance when ill, and counting of new graves in
tor cannot compensate for the failure of unmodified SARS
such locations indicates that the death toll is higher than offi-
to infect humans efficiently is unknown. It may be signifi-
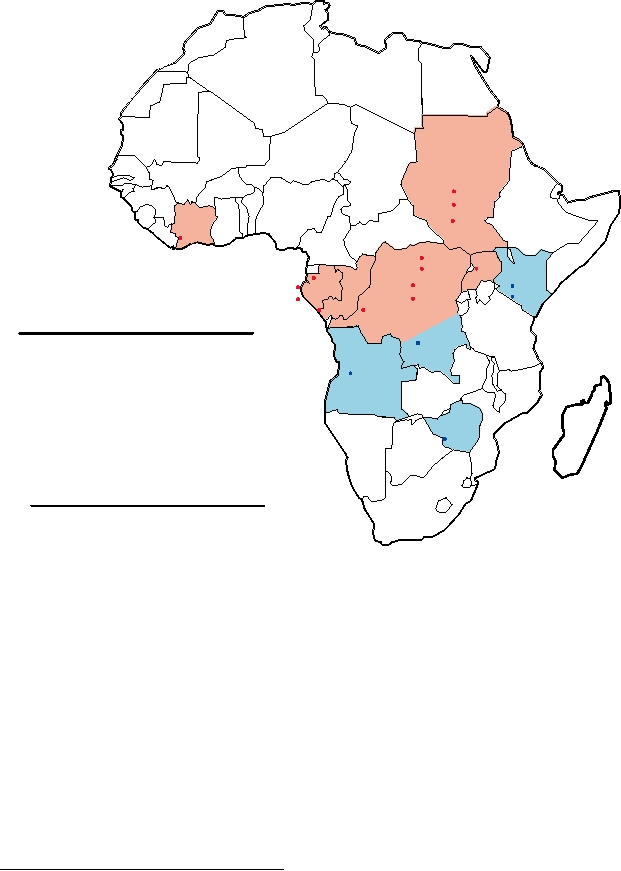
cially reported. The locations of these outbreaks are shown
cant that another human coronavirus, NL63, only recently
on the map in Fig. 8.3. The reported fatality rate in the larger
discovered, also uses ACE2 as its receptor. Other corona-
outbreaks was 8090%.
viruses use different receptors, including aminopeptidase N
Sudan
1976
1979
Ivory
2004
Coast
1994
1976 Uganda
1977
2000 Kenya
1994
Marburg
2003/04
Gabon 1995/96 Congo
1980
1987
2005
1996/97
Year
Cases/
Country
2002
1995
Zaire*
%Mortality
1975
3 (33%)
Zimbabwe
1998/00
Angola
1980
2 (50%)
Kenya
2005
1987
1 (0%)
Kenya
1998/00
149 (83%)
Zaire*
2005
252 (90%)
Angola
Zimbabwe
1975
Ebola
Year
Cases/
Country
%Mortality
1976
284 (53%)
Sudan
1976
318 (88%)
Zaire*
1977
1 (100%)
Zaire*
1979
34 (65%)
Sudan
1994
44 (64%)
Gabon
1994
1 (0%)
Ivory Coast
1995
315 (77%)
Zaire* (Kikwit)
1995/96
37 (57%)
Gabon
1996/97
60 (75%)
Gabon
2000
425 (53%)
Uganda
2002
122 (81%)
Gabon/Congo
2003/04
178 (89%)
Zaire*
2004
17 (41%)
Sudan
2005
12 (75%)
Zaire*
*Now called Democratic Republic of Congo
FIGURE 8.3 Map of Africa showing the different filovirus outbreaks. Data from Porterfield (1995) p. 320, and later
data from Georges-Courbot et al. (1997); Peters and Khan (1999), and news bulletins from the World Health Organization
(2005) at: http://www.who.int/disease-outbreak-news/. Note that in recent years, outbreaks of Ebola disease have occurred
almost annually in the center of the range, particularly in Gabon and the Democratic Republic of Congo. On the contrary,
the recent epidemic of Marburg in Angola was the first in 5 years.
African Ebola Virus
there was an epidemic in Kikwit, Zaire, that resulted in at
Ebola virus was first isolated during a 1976 epidemic of
least 315 cases with >75% mortality. This was followed by
severe hemorrhagic fever in Zaire and Sudan and named
several deaths in western Africa that resulted from consump-
for a river in the region. During this epidemic, the more
tion of a monkey that had died of Ebola. Then there was a
than 600 cases resulted in 430 deaths and asymptomatic
prolonged series of smaller outbreaks in Gabon from 1995
infection appeared to be rare. One case of Ebola occurred in
through 1997. In 2000, Ebola appeared in Uganda for the
1977, and in 1979 there were 34 cases with 22 deaths in the
first time and caused an epidemic of more than 425 cases.
Sudan. In this latter epidemic, an index case was brought to
There have been further outbreaks in 2002, 2003, 2004, and
the hospital and the virus spread to four people there, who
2005 in various countries including Gabon, the Democratic
then spread it to their families. After this, Ebola disease in
Republic of Congo, and Sudan. A map showing these vari-
Africa disappeared until 1994. In late 1994, a Swiss etholo-
ous filoviral outbreaks is shown in Fig. 8.3. Three strains
gist working in the Ivory Coast performed necropsies on
or species of African Ebola viruses are now recognized
chimps. She contracted Ebola but survived, and a new strain
which differ in their virulence. Zaire ebolavirus is the most
of Ebola was isolated from her blood. Then, in May 1995,
virulent with a case fatality rate approaching 90%, Sudan
Search WWH :