Nine of the species shown in Table 7.15 cause genital
Because of the association with cervical cancer, efforts are
warts (species 26 infects both mucosal and cutaneous sur-
being made to develop vaccines. A vaccine directed against
faces). Genital warts are among the most common sexually
HPV-16 and HPV-18 has been shown in clinical trials to be
transmitted diseases. One study found that 46% of college
100% effective in preventing infection by these two viruses,
women examined were positive for HPV DNA in the genital
and also 92% effective against low-risk HPVs. On June 8,
tract. Older women have a lower incidence of HPV, either
2006 the Merck vaccine "Gardasil" was approved by the
because they have had fewer recent partners or because they
Federal Drug Administration for 9- to 26-year-old females.
have acquired some immunity. In many cases the infec-
This quadrivalent vaccine contains antigens from HPV types
tion is cleared completely after some months, but the virus
16, 18, 6, and 11. Widespread use of such a vaccine will
may remain in a latent or persistent form in apparently nor-
result in preventing the majority of cervical carcinomas as
mal tissue adjacent to the wart and the lesions may recur.
well as vocal cord warts caused by HPV, although how long
Immunosuppression results in an increased incidence of
the immunity will last is as yet to be determined.
warts.
A second possible approach to the control of papilloma-
viruses is the use of human α-defensins to block infection
Genital HPVs are clearly associated with cervical can-
cer. Cancer is a rare complication of HPV infection that may
by the virus. These are small peptides secreted by humans
take decades to develop and it requires additional genetic
that have bacteriocidal and antiviral activity (Chapter 10).
mutations. Because of the prevalence of HPVs, however,
Certain of the defensins block infection by papillomaviruses
there are about 500,000 new cases of cervical carcinoma
at high concentrations and, interestingly, in some women
diagnosed annually worldwide and most, perhaps all, are
such concentrations of these peptides occur naturally in the
associated with HPV. In developed countries with a high
genital tract. Application of these peptides could ablate virus
standard of health care, cervical cancer accounts for about
infection, a possibility that is perhaps even more significant
7% of cancer in women, but in developing countries cervi-
because the use of condoms affords very little protection
cal cancer accounts for 24% of all cancer in women. In the
against papillomavirus infection.
United States about 4000 deaths occur annually from cervi-
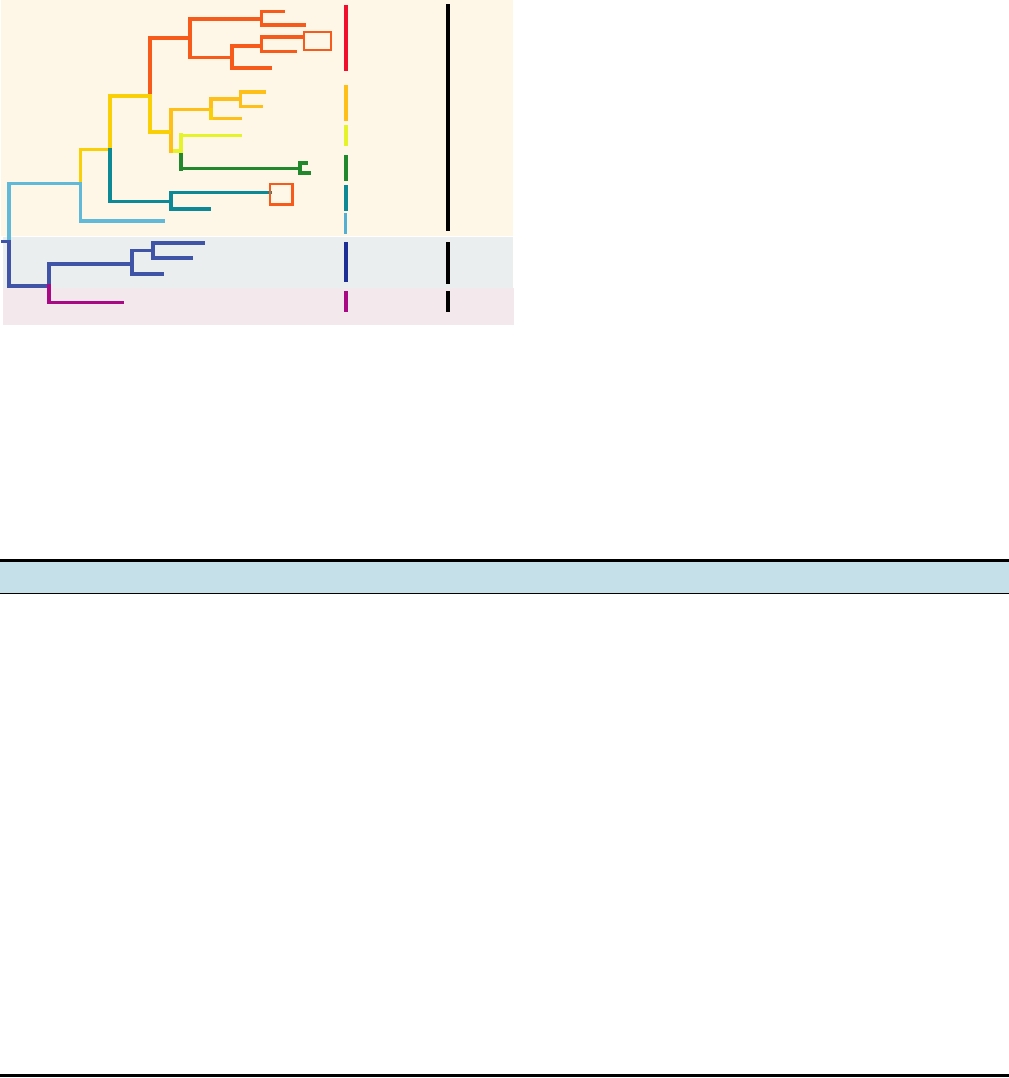
A phylogenetic tree of selected human papilloma viruses
cal cancer.
constructed using sequences in the E2 gene is shown in
Genital HPVs can be divided into low risk (rarely or
Fig. 7.32. There is no simple relationship between the relat-
never associated with cancer), intermediate risk, and high
edness of the different viruses as illustrated by this tree and
risk (often associated with cancer). HPV-16 accounts for
the target tissue infected by the viruses or the risk of neo-
about half of the cancers, and HPV-16 and HPV-18 together
plastic transformation following infection by them. Note, for
account for more than 70% of the cancers. As described
example, that HPV-16 and HPV-18, which cause the major-
earlier, papillomaviruses encode oncoproteins that interfere
ity of cervical carcinoma, are widely separated in the tree,
with the functions of tumor suppressor proteins, and the
although both are alphapapillomaviruses.
high-risk HPV-16 and HPV-18 interfere most strongly. The
high-risk viruses also induce the production of telomerase.
FAMILY PARVOVIRIDAE
These activities are almost certainly the basis of papilloma-
virus-induced cancer, but development of tumors requires
more than just the expression of these genes. Integration of
The parvoviruses are small icosahedral viruses that are
the HPV genome into the host chromosome, or at least that
1826 nm in diameter (Figs. 2.1 and 2.5). They contain
part which encodes E6 and E7, occurs and this integration
ssDNA of about 5 kb as their genome. Different viruses
results in higher expression of the viral transforming genes.
variously package the minus strand (the strand complemen-
In addition, these viruses destabilize host chromosomes
tary to the messenger sense) or a mixture of plus and minus
so that chromosomal abnormalities occur, including aneu-
strands. Two subfamilies are recognized. The Densovirinae
ploidy. Thus progression to a cancer is a long-term proc-
are viruses of insects and consist of three recognized gen-
ess that requires many changes for the transformed cell to
era. The Parvovirinae are viruses of birds and mammals
become immortalized and invasive.
and five genera are recognized at present. A partial listing
HPV types 6 and 11 (two types belonging to species 6),
of the members of the Parvovirinae is shown in Table 7.16.
which cause genital warts, may also infect the mouth, nasal
These viruses are species specific and also specific for the
cavity, larynx, or lungs. Infection of the larynx may be prob-
spectrum of tissues that can be infected. Unlike other DNA
lematic because of the resulting obstruction of the airways
viruses, the parvoviruses do not encode genes that induce the
or because of hoarseness caused by infection of the vocal
cell to enter S phase, and they can only replicate in cells that
cords. Surgical removal of the papillomas may be required.
are actively replicating. The members of the Dependovirus
These papillomas tend to recur, requiring further operations.
genus are further limited in their replication in that normally
There are in addition two HPVs known that infect only the
they can only replicate in cells that are infected by an adeno-
oral cavity.
virus, a herpesvirus, or a papillomavirus.
Strains
Species
Genus
Benign/Malignant progression
Site of
Infection
33
58
Squamous intraepithelial dysplasias
Mucosal
HPV16
16
Carcinoma of squamous exocervix, penis, esophagus
31
35
11
Benign genital warts and laryngeal papillomas
Mucosal
HPV6
6
13
Alpha
HPV32
Benign oral focal epithelial hyperplasia
Mucosal
42
2
HPV2
Benign common warts
Cutaneous
57
18
HPV18
Adenocarcinoma of glandular endocervix
Mucosal
39
Small cell neuroendocrine carcinoma
HPV26
51
47
Epidermodysplasia verruciformis (EV), benign warts
Beta
5
Cutaneous
HPV5
Some progression to squamous carcinoma
8
HPV1
Mu
Benign plantar warts
Cutaneous
1
FIGURE 7.32
Phylogenetic tree of the human papillomaviruses based on the nucleotide sequence of the amino-
terminal half of the E2 gene. The sites of infection and the potential for neoplastic progression are shown. The numbers of
the strains are shown (compare Table 7.15), the strains are grouped into species according to new taxonomy, and the two
high-risk viruses that cause most cases of cervical carcinoma are boxed. Note that there is no simple relationship between
position on the phylogenetic tree and either the site of infection or probability of progression to malignancy, although the
two viruses causing the most malignancies are both in the Alphapapillomavirus genus. Adapted from Nathanson et al.
(1996) p. 273 and taxonomic data from Fauquet et al. (2005).
TABLE 7.16 Parvovirinae
Genus/members
Host(s)
Transmission
Disease
Parvovirus
Minute virus of mice
Mice
Contact, fomites
?
Feline panleukopenia
Dogs, cats
Contact, fomites
Enteritis in adults, myocarditis in pups
Kilham rat parvovirus
Rats
Stillbirth, abortion, fetal death, mummification
Porcine parvovirus
Swine
Erythrovirus
B19
Humans
Fifth disease, aplastic anemia, hydrops fetalis,
Several primate parvoviruses
arthritis immunodeficiency
Dependovirus
Adeno-associated virus 15
Humans
Transplacental (AAV-1), vertical (AAV-2)
None
Adeno-associated viruses of
Cattle, dogs,
None
other species
sheep
Goose parvovirus
Geese
Vertical transmission
Hepatitis
Amdovirus
Aleutian mink disease
Mink
Contact, fomites
Chronic immune complex disease
Bocavirus
Bovine parvovirus
Cattle
Enteritis
Human bocavirus
Humans
Respiratory infections
Transcription of the Viral Genome
structural or replication gene, referred to as NS or REP, is
located at the 5′ end of the plus-sense copy of the genome
The genome organizations and transcription maps for two
human parvoviruses belonging to different genera are shown
and the gene for the capsid proteins, referred to as VP or
CAP, is located at the 3′ end. The two genes are present in
in Fig. 7.33. The parvovirus genome contains two genes,
each of which is transcribed into multiple mRNAs. The non-
the same orientation so that only one strand is transcribed.
AAV-2 (Dependovirus)
Structural protein(s)
3'
5'
Replicase
DNA
P19
P40
P5
A1A2
mRNAs
Proteins
CAP
An
1
Rep 78
CAP
An
2
Rep 68m
CAP
An
Rep 68M
3
CAP
4
An
Rep 62
An
Rep 40m
5
CAP
An
Rep 40M
CAP
6
7
?
An
CAP
VP1
VP1
An
CAP
8
VP2
VP3
CAP
An
VP2,VP3
9
B19 (Erythrovirus)
Structural protein(s)
3'
5'
Replicase
DNA
P
A1A2
A3
A4
mRNAs
Proteins
VP1
CAP
An
1
7.5k,VP1
VP1
CAP
An
2
VP1
CAP
An
3
NS1
VP2
CAP
7.5k,VP2
4
An
VP2
CAP
An
5
VP2
CAP
An
6
7.5k
CAP
An
7
?
CAP
7.5k,11k
8
An
CAP
11k
An
9
0
1
2
3
4
5
kbp
Promoters
Splice
Adenylation
ORFs
Terminal
Acceptors
Sites
Palindromes
1
2
3
P5
A1 A2
FIGURE 7.33 Genome organizations and transcription/translation schemes for two human parvoviruses belonging to
two different genera. Adeno-associated virus (AAV) is a dependovirus and B19 virus is an erythrovirus. These viruses
use 3 and 1 promoters, respectively, to make a set of spliced and unspliced messages, all transcribed from one DNA
strand, from which the various virus proteins are translated. Terminal palindromes are shown as shaded boxes. Adapted
from Heegaard and Brown (2002), Figure 2; Mouw and Pintel (2000), Figure 1.
same eight-stranded antiparallel β sandwich present in many
B19 has only one promoter for initiation of transcription but
RNA and DNA viruses (Chapter 2), which suggests that these
two poly(A) addition sites, whereas AAV has three promot-
various capsid proteins share a common ancestry.
ers for transcription but only one poly(A) addition site. The
The parvoviral genome is packaged into preassembled cap-
use of multiple promoters or poly(A) sites is combined with
sids starting from the 3′ end. Packaging requires the helicase
alternative splicing events to give rise to a number of mRNAs.
activity of NS/REP and the expenditure of ATP. In viruses for
The best understood translation products are a nonstructural
which the two ends of the genome are the same, equal numbers
protein of about 80 kDa and the two or three capsid proteins.
of DNA genomes of both plus and minus orientation are made
and packaged. However, in viruses whose genomes have dif-
Replication of the Viral DNA
ferent palindromic sequences at the two ends, only the minus
sense genome is packaged. Thus, a packaging signal at the
Replication of parvoviral DNA occurs in the nucleus.
3′ end is specifically recognized for packaging. In experiments
Replication of the DNA and transcription of mRNAs are
in which a virus that normally packages only the minus-sense
effected by host DNA and RNA polymerases but the NS or
genome is induced to package both plus- and minus-sense
REP protein of the virus is required. The activities of this
strands by changing the 3′ end sequence of the plus-sense
protein include a site-specific DNA-binding activity, a site-
strand, it was found that the plus-sense genome was packaged
specific DNA nuclease activity, and helicase activity.
poorly and got hung up after about half of the genome was
Parvoviral DNA is linear and possesses palindromic
packaged. Thus, the minus-sense DNA has evolved so that
sequences at the two ends. In some viruses the palindromic
secondary structures that form in single-strand nucleic acids do
sequences at the two ends are the same, whereas in other
not interfere with packaging, whereas the plus-sense genome,
viruses they are different. These palindromic sequences are
not being subject to such selective pressure, has secondary
100300 nucleotides long, depending on the virus, and can
structures within it that preclude efficient packaging.
fold back to form a very stable hairpin structure, as illustrated
in Fig. 7.34 for two different parvoviruses. The hairpin primes
DNA replication, with the 3′ end serving as a primer that is
Genus Erythrovirus
elongated to form a double-stranded intermediate, as illus-
trated in Fig. 7.35. How this intermediate is used to continue
B19 virus, the only known human virus in the genus
DNA replication and how it is resolved to give plus and minus
Erythrovirus, was until recently the only recognized human
DNA genomes is not clear, although it is known that the viral
pathogen among the parvoviruses. Infection of humans
nonstructural protein is involved. Models have been proposed
with B19 is accompanied by nonspecific flulike symptoms
that involve either continued rolling of the hairpin or the for-
followed by symptoms of erythema infectiosum (fifth dis-
mation of cruciform structures that might be resolved by cel-
ease), which presents as a generalized erythematous rash
lular recombination enzymes. In the favored model, the rolling
with a "slapped cheek" appearance and inflammation of
hairpin is resolved by an endonuclease activity in NS/REP, as
joints. Children infected by the virus are usually not very ill.
illustrated schematically in the figure, an activity known to
However, illness in adults can be more serious because the
be present in the protein. Resolution in this way results in the
joint inflammation may mimic rheumatoid arthritis and can
flipping back and forth of the terminal sequence. Such flip-
persist for months or years. In addition, virus infection of
ping is known to occur for at least some viruses, which results
people with some forms of anemia can be quite serious.
in the palindromic sequence being present in two orientations
B19 has a tropism for human erythroid progenitor cells,
that are distinguishable. In some viruses, only one end of the
which are rapidly dividing cells capable of supporting virus
genome flips, whereas in others both ends flip.
replication. The virus is cytolytic and the infected cell dies.
Thus, infection of erythroid progenitor cells results in a sup-
pression of erythropoiesis for 57 days following infection
Assembly of the Virion
by the virus. In healthy humans, whose red blood cells last
The parvovirus virion is a T=1 icosahedron that is con-
for 160 days, this is a not a serious event. However, in people
structed of 60 molecules of capsid protein (see Fig. 2.5). The
suffering from chronic anemias the inability to synthesize
major capsid protein is called VP2 and is about 60 kDa in size.
red blood cells for a week may be serious and occasionally
Smaller amounts of a larger capsid protein called VP1 (about
fatal. In particular, patients with hemolytic anemia have a
80 kDa) are present in all parvoviruses, and some contain in
low hemoglobin concentration in the blood because their red
addition a third capsid protein called VP3. The two or three
blood cells have a short life span, only 1520 days, so that
structural proteins share significant sequence overlap, being in
arrest of erythropoiesis in the bone marrow leads to a sharp
essence variously truncated forms of the largest protein (Fig.
fall in hemoglobin concentration and worsening symptoms
7.33). The structure of the major capsid protein of a parvovi-
of anemia. Other populations at increased risk following
rus has been solved to atomic resolution and it possesses the
B19 infection include patients with compromised immune
A
Structural Protein(s)
5
3
Replicase
B
AAV A
MVM
T
A A
T T
G
C
AC
50
C C
G C
AA
G G
70 C G
G A
50
C G
G G
C
T G
C
G G
G C
GG
C C
G C 80
C
G G
C
C
C G G C
C
T
G G
G G 70
C G C C
C
C
G G
C
G
C CT
C
G
A
T
G
C
C G
TT
A
40
G C
G C
G
C
40
A T
T
A
G C
G
C
T A 90
C
G
C G
A
T
A T
C 80
G
C G
T
A
T A
G
C
C G
A
T
30
G C
A
T
C G
30
T
A
T A
G
C
C G
C
G
G C 100
A
TA
C G
G
A 90
T A
A
G
C G
T
A
G C
G
C
C G
T
A
20
G C
A
T
C G
20 C
G
G C
C
G
T A
C G 110
A
T
T A
A
T
C G
100
C
G
T A
C
G
C G
A
T
C G
G
C
C G
10
T
A
T A
C
G
C G
10 A
T
T A
A
T
C G 120
G
C
C G
A
T
G C
110
T
A
G C
T
A
T A
T
A
130
140
T A
T
A
AGGAACCCCTAGTGATGGAG.
..
.
120
T
A
A
T
3
3
ACCGCTTATC.
...
FIGURE 7.34 Stable hairpin structures predicted from the palindromic sequences at the 3′ termini of parvovirus
virion DNAs. (A) Diagram of the genome, showing the location of the hairpin. (B) The most stable secondary structure
predicted by the 3′ terminal nucleotide sequences of MVM DNA and AAV DNA. Adapted from Fields et al. (1996)
p. 2175.
5'
3'
d
A
cb
a
A BC aD
Virion DNA
Hairpin formation
C
c
A BCaD
aD
dA
d A c b a
a
A3'
b
B
Elongation
c
C
A
aD
d
a
A
B
b
Resolution
C
Acb
a
d
a D
A d
D a C B A 3'
B
Nick at green arrowhead
Elongate from nick
d Acba
3' A
cb
a D
D
aCBA
aC
B A D
Separate strands and
form hairpins for next round
FIGURE 7.35
Model for DNA replication of AAV, a dependovirus. This is a modified "rolling hairpin" model, and
results in inversion of both of the repeated sequences at the termini. Models for replication of the autonomous parvoviruses
like MMV are more complex, since in that case the 5′ terminal sequence of the virion DNA is inverted during replication,
while the 3′ terminal sequence is not. Adapted from Brister and Muzyczka (2000).
systems (in which infection by B19 can result in persistent
previously infected, and in the elderly this rises to more than
anemia) and pregnant women. Congenital infection with
90%. Infection of healthy people with normal immune sys-
B19 can be serious and can lead to fetal abnormalities or
tems leads to a solid immunologic response and subsequent
death, due to arrest of red blood cell formation and conse-
immunity to the virus.
quent anemia at critical times during development.
The receptor for the B19 virus is erythrocyte P antigen.
Genus Dependovirus
This antigen is expressed on cells of the erythroid lineage,
but only precursor cells can be productively infected. Mature
The genus Dependovirus contains viruses that can repli-
erythrocytes are terminally differentiated, lack a nucleus, do
cate without a helper under certain conditions and in certain
not divide, and cannot support virus replication. In addition
tissues, but these viruses normally depend upon a helper
to possessing a receptor that allows the virus to enter, other
virus for replication. Known helper viruses include adeno-
factors required for replication are also furnished by ery-
viruses, herpesviruses, and papillomaviruses, but because
throid precursor cells. Transfection of viral DNA into other
the dependoviruses were first found associated with adeno-
cells does not lead to a complete replication cycle and the
viruses, they are called adeno-associated viruses or AAVs.
pattern of RNA transcription differs from that in permissive
AAVs of humans and of numerous other vertebrates are
cells shown in Fig. 7.33.
known. More than 90% of human adults have antibodies
B19 is a common virus. About 50% of adults have anti-
to AAV, which shows that the virus is widely distributed
bodies against the virus, which show that they have been
and common.
Search WWH :