sion molecules that are members of the Ig superfamily,
Most promoters reside outside the open reading frames, but
called nectin-1 and nectin-2, and specific sites in certain iso-
some are found within them. The genome organization is
forms of heparan sulfate. In the case of EpsteinBarr virus,
complex. Some genes lie within other genes, and for a few
a protein called complement receptor 2 (also referred to as
genes both strands of the DNA are transcribed into RNA.
CD21) serves as an accessory receptor and entry receptors
The use of host RNA polymerases to transcribe mRNAs
include HLA class II molecules. Entry of herpesviruses may
limits the host range of a herpesvirus, since cells that do
occur by fusion with the plasma membrane or fusion with an
not express the factors required for recognition of the viral
endosomal membrane, depending on the virus and the cell
promoters are not susceptible to a complete replication
type. Fusion requires the presence of cholesterol in the viral
cycle. The mRNAs are polyadenylated following the cellu-
membrane in at least some herpesviruses, a requirement also
lar AATAAA consensus poly(A) signal and exported to the
shown by alphaviruses, influenza virus, and HIV-1.
cytoplasm for translation. From most transcripts only one
Herpesviruses contain multiple glycoproteins in their
protein is translated. Most mRNAs are not spliced, but some
envelope, and fusion requires the activity of several of these
transcripts are spliced or multiply spliced and partially over-
glycoproteins. Thus, for HSV, the activity of 4 of the 12 or
lapping transcripts may exist that form nested sets, using
more glycoproteins in the envelope are required for fusion.
a common polyadenylation signal. Many of the proteins
synthesized are dispensable in cell culture and function to
extend the host range and tissue tropism of the virus or to
subvert host antiviral defenses.
REPLICATION OF HERPESVIRUSES
Herpes Simplex Viruses (HHV-1 and -2)
The best studied herpesvirus is herpes simplex virus type
1, which has been used as a model for the entire family,
Herpes simplex virus (HSV) causes fever blisters or cold
and a more detailed description of its replication will be
sores around the lips or in the genital area. The disease caused
presented in the section that discusses this virus. Here some
by the virus has been known for thousands of years. The char-
generalities of the replication cycle of herpesviruses are
acteristic vesicles were described by the ancient Greeks and
described.
have been noted in writings through the ages. The name of the
It has long been thought that herpesvirus DNA repli-
virus comes from the Greek word herpes, to creep or crawl,
cates by a rolling circle mechanism as illustrated in Fig. 1.9
referring to the lesions caused by the virus. HSV exists as two
Although the DNA is linear in the virus, there is evidence
serotypes, HSV-1 and HSV-2, also known as HHV-1 and
that it has no ends in the infected cell, indicative of circu-
HHV-2. These alphaherpesviruses share about 50% sequence
larization. As described in Chapter 1, a circular genome
identity and produce a similar disease, but usually infect dif-
obviates the need for a special mechanism to repair the ends
ferent parts of the body. HSV-1 infects the facial area, whereas
of the DNA during replication. Recent data suggesting that
HSV-2 infects the genital area. In humans, the viruses lytically
during productive infection, as opposed to latent infection,
infect epidermal and mucosal cells; they latently infect neu-
the DNA remains linear have not been confirmed and the
rons. In the laboratory they lytically infect cells of many differ-
favored hypothesis remains that replication of the DNA is
ent origins and will infect many experimental animals. Because
by a rolling circle mechanism.
of the relative ease of experimental manipulation, HSV has
Replication of herpes DNA occurs in the nucleus, and all
been intensively studied as a model for the entire group of
herpesviruses encode a large number of enzymes that are
herpesviruses. The large size of the genome, 150 kb, and the
involved in nucleic acid metabolism. Among these proteins
large number of encoded genes have made a detailed under-
are a DNA polymerase and a protein that binds to the viral
standing of the virus genome organization very complicated.
origins of replication in order to initiate DNA replication.
However, due to the efforts of large numbers of workers, maps
Herpesviruses encode more than 70 proteins. The pro-
such as that shown in Fig. 7.14 can now be constructed. This
moters used to transcribe mRNAs fall into different classes
map illustrates the different genes of HSV, their functions, and
such that there is a temporal program for the expression of
their classification into three temporal groups described later.
genes during the lytic cycle. The proteins encoded by the
The complexity of the genome and the existence of very many
first genes to be expressed, the immediate-early genes, have
genes are clear from this diagram.
regulatory functions. Expression of these genes permits the
expression of the early genes, most of which are involved
Lytic Infection by the Virus
in DNA replication. Expression of the early genes, in turn,
permits the expression of the late genes, most of which
A schematic representation of the lytic cycle of herpesvirus
encode structural proteins required for assembly of virions.
infection is shown in Fig. 7.15. Infection is normally initiated
The mRNAs are transcribed by host RNA polymerase II and
by fusion of the viral membrane with the cell plasma mem-
the promoters are in general 40120 nucleotides in length.
brane. The nucleocapsid is then transported to nuclear pores
US8.5 γ2
US9
US4 γ
US10
US11 γ2
US5
US6 γ1 α47
22
US7γ
O α
c a
b
riS
US8 γ2
8β
α
-4
OR
γ
10 γ
3 F
US
α-4.5 γ P
0.0
1
1 β
0.1
0
12 γ
13 14
150 0
LAT
10
15A γ6
140
56
1
20
γ
17
kilobases/
55
130
15B γ
30
map units
18 γ
54 α
120
53 γ
40
Major Capsid
19 γ1
52 β
110
Protein
50
51 γ
20 γ
100
50 β
21 γ1
60
γ2
49.5 γ
90
80 70
2
UL
23 β 2 γ2
9 γ
4
48 2
0.6
γ
24
0.5
25 γ
47
γ
2 γ
46 2
26 6 γ
γ γ2
.5
45 44 β
γ
27
43
γ1
31 γ2
32 γ2
33
34
35 γ2
36 γ2
Virion Proteins
Essential
Non-essential
Other Non-essential
Regulation
Latency
DNA Synthesis
Host Range
Capsid/Assembly/Virion
Unknown Function
Envelope (Glycoproteins)
Other Enzymes
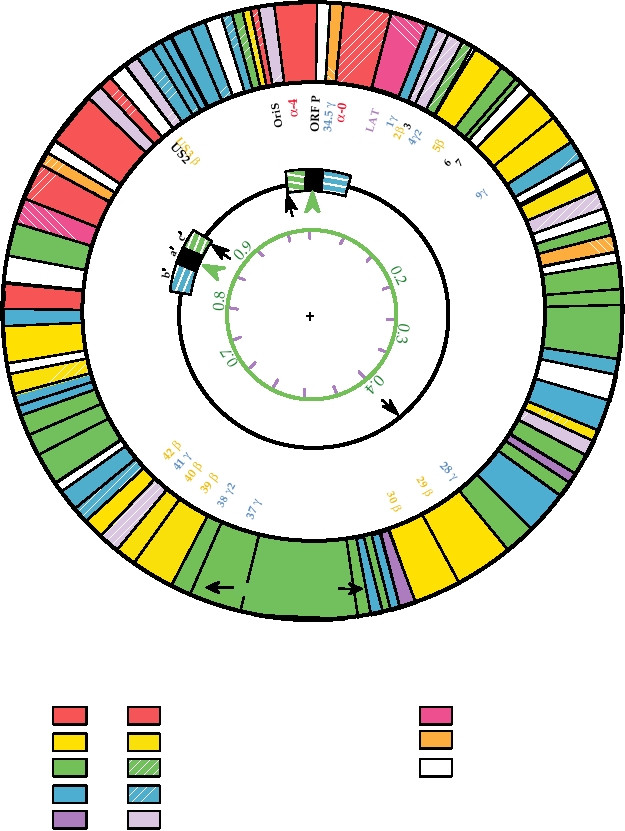
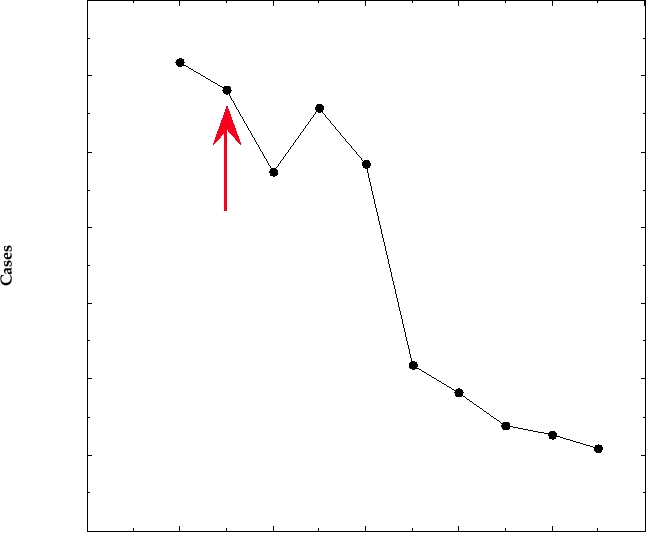
FIGURE 7.14 Functional organization of the HSV-1 genome. Circles are described from the center outwards. Circle
1 shows kilobase pairs in black and map units in green. Circle 2 shows the general organization of the gemone: UL, the
long unique region; US, the short unique region; and a, b, c, the inverted repeats. The black arrows are the three origins
of DNA replication, and the green arrowheads show the sites of cleavage/linearization of concatameric or circular DNA.
Circle 3 identifies the ORFs, color coded according to their kinetic class (a in red, b in ochre, g in blue). Black numbers
are ORFs not belonging to a particular class. The outer circle indicates the functions of the ORFs, where known, by color
coding as indicated in the key below. Solid colors indicate ORFs required for replication in tissue culture cells, while the
corresponding patterned ORFs can be deleted without affecting replication in culture. Only one of the two copies of α-0
(diagonal red stripes) is required. Adapted from Fields et al. (1996) p. 2245.
where the viral DNA is released and enters the nucleus (stages
octomer sequences, and, to simplify somewhat, a complex of
13). There the viral DNA is transcribed by RNA polymer-
Oct-1, TIF, and another cellular factor called C1 binds to the
ase II to produce mRNAs. Of the 80 or so mRNAs produced,
consensus sequence TAATGARAT in the HSV genome (R=A
only 4 appear to be spliced. Transcription is regulated and can
or G) (stage 3). This binding results in transcription of the
be divided into three phases, α, β, and γ. Transcription of α
five α genes (stage 4) that are translated into proteins called
genes, also called immediate-early genes, occurs immediately
ICP0 (ICP = intracellular protein, to distinguish them from
on entry of the DNA into the nucleus. Transcription of these
virion proteins), ICP4, ICP22, ICP27, and ICP47 (stage 5).
genes is transactivated by TIF, a virus-encoded protein that
These proteins have regulatory roles in viral replication and
are required to activate the β genes (stage 6). ICP4 transac-
is present in the tegument of the virion. TIF interacts with a
cellular transcription factor called Oct-1, which recognizes
tivates HSV genes and functions together with ICP0. ICP27
A. Early Events
B. Late Events
1
NUCLEUS
NUCLEUS
9
2
a-TIF
g mRNA
8
Nuclear enzymes
3
translation
a proteins
4
a mRNA
10
b proteins
translation
5
g proteins
11
12
HSV-1 virion
6
b mRNA
translation
Tegument
DNA genome
13
Nucleolus
7
C
Golgi
maturation
EUKARYOTIC HOST CELL
14
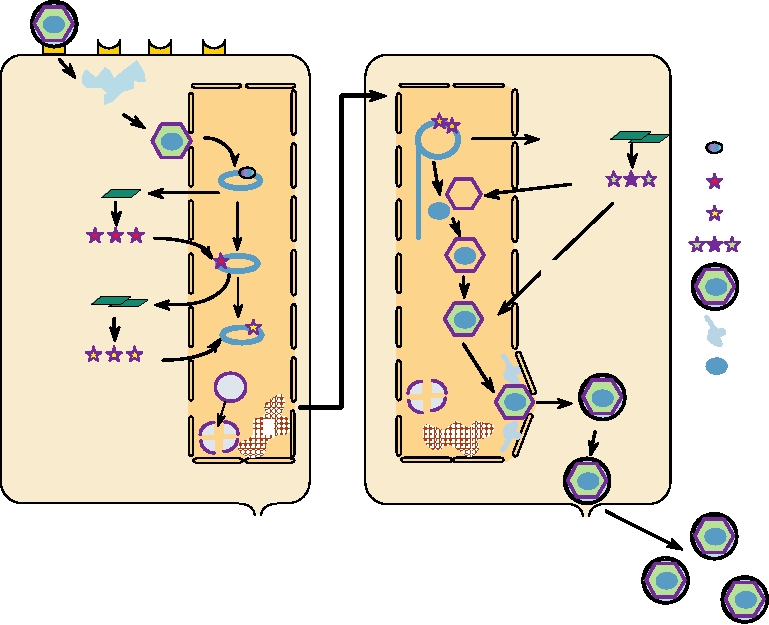
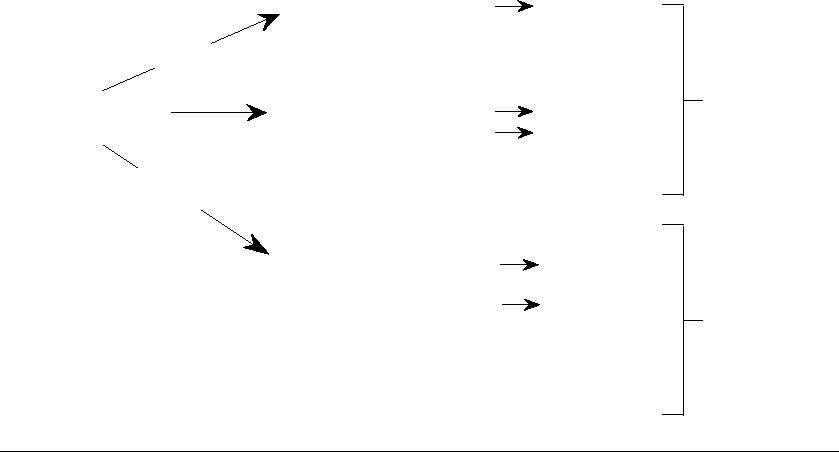
FIGURE 7.15 Replication of a herpesvirus. (A) Early events: Stage (1) attachment to cellular receptor and entry
by pH-independent fusion with plasmalemma; stage (2) release of tegument proteins causing shutoff of host protein
synthesis, nucleocapsid goes to a nuclear pore; stage (3) α-TIF (VP16) is transported to the nucleus; viral DNA enters
nucleus and circularizes; stage (4) transcription of early α genes by nuclear enzymes, and export of α mRNA; stage (5)
immediate early α proteins are transported to the nucleus; stage (6) α proteins are involved in β mRNA synthesis; stage
(7) chromatin (C) is degraded and nucleoli disaggregated. (B) Late events: Stage (8) β proteins replicate DNA by rolling
circle to give head-to-tail concatemers; stage (9) β proteins lead to transcription of late γ mRNAs that are translated into
structural proteins; stage (10) formation of empty capsids; stage (11) packaging of unit-length DNA into capsids; stage
(12) addition of further structural proteins; stage (13) particles receive envelope (and tegument?) at nuclear membrane;
stage (14) particles mature in Golgi and exit by exocytosis. Redrawn from data in Roizman and Sears (1990).
activates expression of β genes and blocks splicing of cellular
nucleotides in length and have a 100- to 200-nt critical core,
pre-mRNAs, thereby interfering with host protein synthesis. It
but only one origin appears to be required. During DNA rep-
shuttles between the nucleus and the cytoplasm at late times.
lication, up to 50% of the DNA in the cell becomes viral.
DNA replication is required for transcription of the γ
ICP22 is not required in cultured cells and its function is
unknown. ICP47 blocks presentation of antigens to cytotoxic
genes, most of which encode the proteins that form progeny
T cells (CTLs) (see Chapter 10).
virions, of which there are more than 30 (stages 9 and 10).
Most of the β genes encode proteins required for DNA
Assembly and release of virions (stages 11, 12, and 13) (see
replication, which include a DNA polymerase, a primase/
also Fig. 2.25A) was described before. Cleavages in capsid
helicase, a DNase, both double-strand and single-strand
proteins occur during assembly, associated with uptake of
DNA-binding proteins, thymidine kinase, ribonucleotide
DNA, and may serve a maturation function.
reductase, dUTPase, uracil DNA glycolase, and a protein
More than half of the 80 or so genes in HSV are not
kinase. The activities of these proteins result in morphologi-
required for replication of the virus in cultured cells.
cal changes in the nucleus, which include fragmentation of
However, all appear to be required for infection of humans
the nucleolus and degradation of the host-cell chromosomes
and maintenance of the virus in nature.
(stage 7), and allow DNA replication (stage 8) to begin.
Host-cell macromolecular synthesis is shut off after lytic
There are three origins of replication, which are 8001000
infection. A component of the virion tegument called vhs
(for virion host shutoff) is an RNase that inhibits host pro-
Primary Infection and Maintenance of the Virus
tein synthesis by degrading cellular mRNA, so that inhibi-
Primary infection is established when a seronegative indi-
tion begins very early. The enzyme appears to show some
vidual comes in contact with the virus, usually from a person
specificity in the mRNAs degraded, as some mRNAs are
who is secreting the virus at the time. Thus, a person with
degraded rapidly, some more slowly, and some not at all.
a reactivated infection can serve as the source of infection,
This enzyme also accelerates the turnover of viral mRNAs,
but virus may be actively secreted even when no lesions are
helping the transition from one stage of the infection cycle to
present. Acute infection is accompanied by the formation of
the next. At late times after infection, the enzyme is rendered
vesicles that are sites of virus replication and contain infec-
inactive by another viral protein. Later synthesis of new viral
tious virus. In the case of HSV-1 these vesicles are normally
proteins leads to a more profound inhibition of host expres-
found in the facial skin and in the oral mucosa, and latent
sion, due in part to the fragmentation of the nucleolus and
infection is established in the trigeminal ganglion. HSV-2
degradation of the host cell chromosomes. These events
is sexually transmitted and infects the genital area. Latent
invariably result in the death of the host cell.
infection is established in neurons in the sciatic ganglion. At
one time, HSV-2 was thought to be a causative agent of cer-
Latent Infection
vical carcinoma, but this disease is now known to be associ-
ated with papillomavirus infections, which are also sexually
After the infection of epithelial tissues, HSV infects sen-
transmitted and produce warts in the genital region.
sory nerves that serve these tissues and establishes a latent
HSV-1 is extremely common in human populations. It
infection. Virus, probably as nucleocapsids, is transported
has been found in every population examined, with from 50
up axonal processes to sensory ganglia, where it is esti-
to more than 90% of adults having been infected. Primary
mated that 510 copies of viral DNA, in the form of circular
infection often occurs early in life, but may be delayed in
episomes, take up residence. Less than 1% of the neurons
some fraction of the population, especially in developed
within a ganglion appear to be latently infected. Much of
countries. Because of its mode of transmission, HSV-2
what we know about latent infection comes from studies of
is less common and primary infection occurs later in life.
animal models. HSV will infect and establish a latent infec-
Estimates of its prevalence in human populations center near
tion in mice, guinea pigs, and rabbits, but it is not known
10%, but the only population found to be completely free of
how faithfully these animal models reflect the situation in
antibodies to HSV-2 was a group of Roman Catholic nuns.
humans. Only one viral transcript is detected in latently
The acquisition of seropositivity to HSV-1 and HSV-2 in the
infected neurons, called latency-associated transcript or
United States in different populations is shown in Fig. 7.16.
LAT. This transcript promotes neuronal survival by inter-
fering with apoptosis of infected neurons by means of an
RNA silencing pathway (Chapter 10). The detailed mecha-
Serious Disease Caused by HSV
nisms by which latency is established and maintained, and
of how latency is abolished upon reactivation of the virus,
Primary infection with HSV is usually inapparent or
are not understood. It is assumed that part of the answer is
produces only minor illness. In one study of children, for
that neurons are nonpermissive, or at best semipermissive,
example, 70% of infections were found to be asymptomatic.
for virus replication.
However, HSV infection of neonates is almost always symp-
Reactivation of virus occurs sporadically, in response to
tomatic and frequently fatal. Infection in utero, during deliv-
stressful stimuli such as fever, exposure to UV light, men-
ery, or shortly after birth usually leads to a disseminated
struation, or emotional stress. The frequency of recurrence
infection often accompanied by encephalitis. The neurotro-
varies in different people from monthly to less than once
pism of the virus also leads to serious illness on occasion in
per year. Stresses that are well known to induce reactivation
postneonatal individuals. The Centers for Disease Control
include high fever resulting from infection with influenza
and Prevention estimates that about 50 cases of herpes
virus and prolonged exposure to sunlight. On induction, lim-
encephalitis occur yearly in the United States that are usually
ited replication of virus occurs in the neuron and it travels
fatal if untreated, and this incidence may be underestimated.
down the axon, where it infects epithelial cells served by
Of these cases, about half are due to primary infection and
that neuron. Thus, fever blisters erupt in the same tissues as
half to reactivated infection. HSV keratoconjunctivitis also
were originally infected, and these lesions contain infectious
occurs and can lead to impairment of vision. Herpetic whit-
virus. Classically, these lesions occur around the lips (HSV-1) or
low is an occupational hazard of dentists and other health
in the genital region (HSV-2). Virus replication in epithelial
care workers, characterized by painful herpetic lesions on
cells is quickly controlled by the immune system and the
the fingers. And as is true of many viral infections, HSV
lesions heal within 2 weeks or so. Although not yet resolved,
infection or reactivation can be very serious in individuals
it appears that the limited replication of the virus in the
whose immune function is compromised by suppressive
neuron is probably fatal for that neuron.
therapy for organ transplant or by infection with HIV.
100
80
HSV-1
Black Men
White Men
60
HSV-2
Black Men
White Men
Black Women
40
White Women
20
0
5-9
15-19
25-29
30-39
40-49
50-59
60-74
1-4
10-14
20-24
Age group
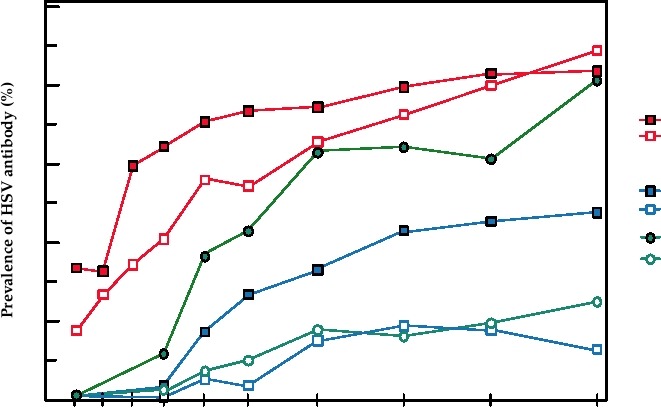
FIGURE 7.16 Seropositivity to herpes simplex virus types 1 and 2 as a function of age, sex, and race in the United
States in 1978. Data for this figure came from Johnson et al. (1989), and Nahmias et al. (1990).
HSV infections can be treated with acyclovir, a guanine
virus by the infected cell for an extended period before the
analogue that is incorporated into DNA and results in chain
host antiviral defenses can shut it down.
termination. It has little toxicity for host cells but inhibits
Establishment of latency in neurons is also an important
the replication of HSV-1, HSV-2, and VZV DNA. It is less
part of the strategy evolved by HSV to avoid host defenses.
effective against other herpesviruses.
Neurons are immunologically privileged, and elaborate
mechanisms to protect the infected neuron for long periods
of time are not necessary. Furthermore, because neurons are
Interference with Host Antiviral Defenses
nonrenewing and long lived, the establishment of latency in
HSV interferes with several host defense mechanisms.
these cells allows the virus to persist indefinitely even in the
These activities will be described in more detail in Chapter
absence of reactivation.
10, in which the host defense mechanisms themselves are
described, but a brief summary of HSV activities is pre-
sented here. The virus interferes with the interferon system,
Varicella-Zoster Virus (HHV-3)
with the lysis of infected cells by cytotoxic T lymphocytes
(CTLs), with complement-mediated lysis of infected cells,
Varicella-zoster virus (VZV, also known as HHV-3) is an
with antibody-dependent lysis of infected cells, and with
alphaherpesvirus that is the prototype member of the genus
the lysis of infected cells by a cell suicide pathway called
Varicellovirus. Other members of this genus infect monkeys,
apoptosis. The antiviral effect of interferon is blocked by
horses, and pigs (see Table 7.7 and Figs. 7.11 and 7.12). The
viral protein 34.5, which causes a cellular phosphatase to
VZV genome is 125 kb in size and contains at least 69 dif-
remove the inactivating phosphate put on eukaryotic trans-
ferent genes, of which all but 5 are homologous to genes in
lation initiation factor eIF-2 (Chapter 10). CTL lysis of
HSV. The homologous genes are almost all colinear with the
infected cells is blocked by protein 47, which inhibits the
corresponding genes in HSV, and thus the gene map of VZV
presentation of peptide antigens to the T cells by major his-
is essentially the same as that for HSV. Molecular studies of
tocompatibility complex class I molecules. Complement-
VZV have been hampered by the inability to produce high
mediated lysis is blocked by HSV proteins that interact
titered virus stocks in cultured cells, because the virus remains
with complement. Antibody-dependent cellular toxicity
cell associated. Where known, however, the VZV life cycle
is blocked by HSV-1 IgG Fc receptors, composed of gE
closely resembles that of HSV, as would be expected from
and gI, that are expressed on the surface of the infected
their close relationship. This close relationship also exhibits
cells. Finally, HSV gene products suppress apoptosis by the
itself biologically: VZV, like HSV, lytically infects a number
infected cell. The net result is to allow the production of
of different cells but most characteristically epidermal cells
resulting in skin lesions, and VZV, like HSV, establishes a
vesicular lesions of shingles contain live virus that can infect
lifelong latent infection in sensory ganglia. The vesicle fluid
children and give them chickenpox. Thus, the virus is able
present in VZV skin lesions contains large amounts of free
to remain latent for decades and then erupt in an essentially
virus and can spread the disease to susceptible persons.
unchanged form to start a new epidemic of chickenpox.
Episodes of zoster are more frequent in older people but
a second episode of zoster in a person is rare. It appears that
Chickenpox
the reactivation of the virus leads to a boost in immunity to
VZV causes two different diseases known as chickenpox
the virus that prevents further episodes. Immunity to VZV
(varicella) and shingles (zoster). Chickenpox is a highly con-
is primarily a function of CTLs. Children that suffer from
tagious childhood disease contracted by contact with other
agammaglobulinemia, who are unable to make antibodies,
children with chickenpox or with an adult with shingles.
have a normal course of infection by VZV, but children that
The virus is transmitted by the aerosols and virus replica-
are deficient in CTL production often die. Furthermore, it
tion begins in the upper respiratory tract. It later dissemi-
has been found that an accelerated CTL response is associ-
nates through the bloodstream to other areas of the body.
ated with asymptomatic infection or a mild disease. Finally,
The characteristic feature of the disease is a rash of vesicu-
reactivation of VZV to produce zoster is correlated with
lar lesions in the skin that are often quite itchy. Up to 2000
decreased CTL responsiveness against VZV, but not with
occur in some patients, but fewer than 300 is the norm. Other
decreased titer of IgG antibodies against the virus.
symptoms include fever, malaise, and loss of appetite.
The disease, which has an incubation period of 1021
VZV in At-Risk Populations
days, is normally self-limited, lasting a week or less, but seri-
ous complications can occur. The most common complica-
Varicella or zoster is a serious illness in people with com-
tion in otherwise healthy children is bacterial infection of the
promised immune systems, and zoster is a frequent compli-
skin lesions, which can become serious. Rare complications
cation in patients undergoing immune suppression or who
include viral pneumonia, central nervous system involvement
have AIDS or leukemia. Before the introduction of antivi-
leading to encephalitis or cerebellar ataxia (loss of muscle
ral drugs, in particular acyclovir, which is fairly effective
coordination during voluntary movements), involvement of
for treatment of VZV infections, children who contracted
the liver leading to hepatitis, or involvement of other organs.
varicella while undergoing immunosuppressive therapy for
Primary varicella infection of adults is a more serious illness
leukemia suffered a very high rate of visceral dissemination
than primary infection of children and complications are more
and pneumonia, with a fatality rate of about 10%.
frequent. Viral pneumonia is not uncommon in adults, and
Primary infection with VZV is also serious in pregnant
adult infection can result in male sterility or acute liver failure,
women, leading to significant mortality in both the mother
albeit rarely, as well as other complications.
and the infant. Congenital varicella syndrome may occur when
infection is in the first trimester of pregnancy, during active fetal
organogenesis. Varicella infection of the neonate is serious as
well, with a high mortality rate in the absence of treatment.
Shingles
Following primary infection, which results in chickenpox,
Epidemiology of VZV
VZV sets up a latent infection in dorsal root ganglia in the
spinal cord, where it may reactivate later in life. Reactivation
The geographical pattern of infection by VZV is peculiar.
is less common than for HSV-1 and is age related, occurring
The virus has a worldwide distribution but infection is much
more frequently in older people, presumably as a result of
more common in temperate regions. In temperate regions,
waning immunity. Reactivation may occur without symp-
infection by VZV is almost universal and occurs mostly in
toms, but most commonly reactivation produces the disease
early childhood, in association with epidemics that have a
known as shingles. Shingles is characterized by painful erup-
peak frequency in winter and spring. In tropical regions,
tions of vesicular lesions in skin, usually in the upper back,
however, only about half of the population contracts chicken-
served by a single sensory ganglion (and therefore the lesions
pox. The difference in attack rate is not due to differences in
do not cross the midline). The disease normally resolves
susceptibility to the virus, because the attack rate is very high
within a few weeks, but neuralgia (nerve pain, from neuro
when uninfected adults move to temperate climates. Why this
= nerve and algia = pain) can continue for up to a year or
difference in attack rates occurs remains a mystery.
more and be quite debilitating. More extensive dissemination
It is interesting to consider the differences in the epidemi-
of the virus occurs in a significant percentage of patients, most
ology of VZV and HSV-1 and the rationale for such differ-
of whom have some underlying immunologic defect or are
ences. HSV reactivates fairly often and the virus is typically
immunosuppressed. Dissemination can result in serious com-
spread to young children by adults with reactivated HSV-1
plications, as described for primary varicella infection. The
when they fondle or kiss the child. There is no requirement
that the virus spread from child to child in order for it to
There is now a live attenuated virus vaccine for chicken-
spread within a population. Because of this, virus perpetua-
pox, licensed in 1995, that was originally developed because
tion does not require large-scale production of virus during
of the severe complications of chickenpox in children
primary infection and primary infection is usually asymp-
undergoing chemotherapy for cancer. Although the disease
tomatic. In contrast, VZV reactivates very infrequently.
is not normally serious and the vaccine is not mandated by
Epidemics may begin with the exposure of a susceptible
the authorities, it has nonetheless been well received in the
child to an adult with shingles, but the major mechanism for
United States. Before 1995, there were 4 million cases of
dispersal of the virus in a population is by epidemic spread
varicella annually in the United States, with approximately
among young children. The requirement for child-to-child
100 deaths and 10,000 hospitalizations each year. Because
spread for perpetuation requires that large-scale virus pro-
chickenpox ceased to be a notifiable disease in 1997, com-
duction occur during primary infection, and this results in
plete statistics on the current incidence of chickenpox in the
the primary infection being symptomatic.
United States are not available. However, 20 states still report
A
1997
Rate per
100,000 population
Not notifiable
DC
No Cases
Guam
< 1.0
CNMI
1.01 to 16.0
Virgin Islands
16.01 to 68.6
Puerto Rico
> 68.6
B
2003
DC
Guam
CNMI
Virgin Islands
Puerto Rico
American Samoa
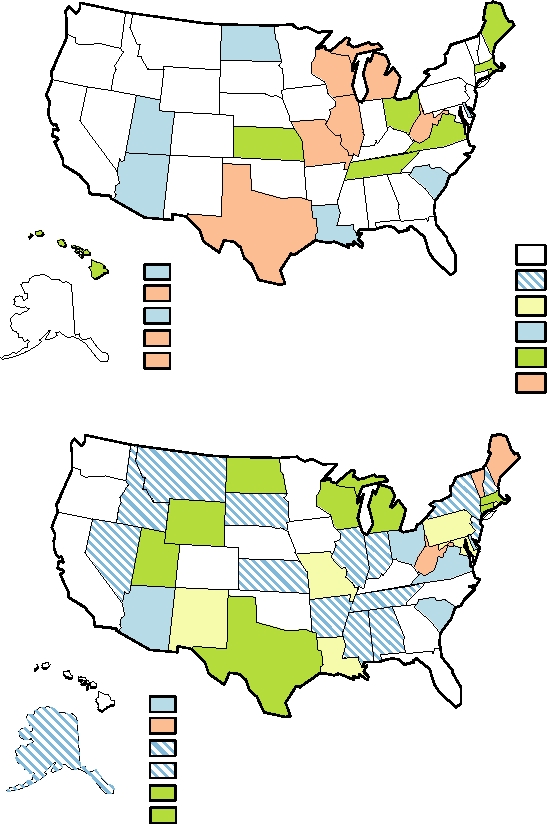
FIGURE 7.17 (A) and (B) Incidence of varicella in the United States in 1997 and in 2003, respectively. In neither year
was varicella a nationally notifiable disease, but some states have active surveillance programs. The color indicates the
incidence rate per 100,000 population. The overall rate for the entire United States was 7.27 in 2003. Data from MMWR,
Summary of Notifiable Diseases-United States for 1998 and 2003.
cases to the Centers for Disease Control and Prevention. The
classified as betaherpesviruses, are also now considered to be
incidence of chickenpox by state for those states that still
gammaherpesviruses. Gammaherpesviruses establish latent
report is shown in Fig. 7.17 for 1997 and 2003 and illustrate
infection in B lymphocytes and cause these cells to prolifer-
the overall decline in the incidence of chickenpox. The
ate. This ability to induce proliferation can result in cancers in
decline is illustrated by year for four states that still report
their native host or in related species.
(Fig. 7.18). In those states, the incidence of chickenpox has
declined by about 80% over a 10-year period. A different
Primary Infection with EBV
formulation of the VZV vaccine has been licensed for adults
EBV infection is virtually universal. More than 90% of
over 60 years old, designed to boost the immune response to
the world's adult population is persistently infected by the
VZV and thus prevent shingles.
virus. Primary infection occurs by transmission of virus
present in saliva. In most human societies, the majority of
EpsteinBarr Virus (HHV-4)
the population is infected by age 3. In developed countries,
EpsteinBarr virus (EBV or HHV-4) is a gammaherpes-
however, infection is often delayed until the teens. Primary
virus. It is named after Tony Epstein and Yvonne Barr, who
infection in infants is normally asymptomatic, but in young
first described the virus in tumor cells from patients with
adults primary infection often results in the disease known
Burkitt's lymphoma. EBV is classified as a member of the
as infectious mononucleosis or the "kissing disease." This
genus Lymphocryptovirus. About 20 viruses infecting Old
disease is characterized by fatigue, fever, rash, and swelling
World primates and about 10 viruses that infect New World
of lymph nodes, the spleen, and, in a minority of patients, the
primates have been identified that also belong to this genus.
liver, for extended periods of time.
Other members of the gammaherpesvirus subfamily belong
Only B cells can be infected by free virus and humans who
to the genus Rhadinovirus and include herpesvirus saimiri (a
cannot produce B cells, a syndrome called X-linked agamma-
virus of squirrel monkeys) and HHV-8, the virus responsible
globulinemia, cannot be infected by EBV. The receptor used
for Kaposi's sarcoma. Equine herpesviruses 2 and 5, originally
to enter B cells is a protein called CD21, a member of the Ig
70,000
60,000
50,000
40,000
Varicella Vaccine
Licensed
30,000
20,000
10,000
0
1992
1994
1996
1998
2000
2002
2004
Year
FIGURE 7.18 Number of reported cases of varicella in Michigan, Rhode Island, Texas, and West Virginia from
1994 to 2003. These states maintained adequate surveillance and in each of the years 1990 through 1995 reported cases
consisting of >5% of their birth cohorts. The number of cases in 2003 represents an 81% decline compared with cases
reported in the 3 years before the vaccine was licensed in 1995. From MMWR, Summary of Notifiable Diseases-United
States, 2003.
superfamily expressed at high levels in B cells. Infection of B
Replication of EBV
cells does not normally result in the production of infectious
EBV will readily infect B cells in culture and estab-
virus, although B cells that differentiate to plasma cells may
lish a latent infection in which a limited set of viral genes
undergo a productive infection cycle. Infection of B cells
is expressed but production of progeny virus is limited.
does result in the stimulation of the cells to proliferate, thus
Although it has been shown recently that epithelial cells, in
expanding the number of infected cells. This proliferation
which the virus undergoes a complete replication cycle, can
leads to a potent T-cell response that controls the number of
be infected by coculture with freshly infected B cells, most
B cells, and it is these B-cellT-cell proliferative cycles that
studies of EBV infection have used B cells.
can result in the symptoms of infectious mononucleosis. As a
The infection of B cells involves complicated interac-
result of T-cell killing of infected B cells, the virus life cycle
tions of the virus with the host cell. Three different forms
changes to establish a latent infection in which a limited set
of latent infection have been distinguished in B cells. These
of viral genes is expressed. Latent infection of memory B
were first described in cells isolated from tumors but can
cells results in lifelong persistence of the virus.
now be reproduced in cultured cells. These different forms
Newly infected B cells are able to transfer the virus
of latency, referred to as latency (Lat) I, II, and III, differ
to epithelial cells in which the virus undergoes a com-
in the extent to which the EBV genome is expressed, as
plete replication cycle, and this appears to be the primary
illustrated in Fig. 7.19. Lat I cells express only one pro-
mechanism by which free, infectious virus is produced.
tein, EBNA1 (EpsteinBarr nuclear antigen). They also
Infection of cells in the oral mucosa results in virus being
express RNAs that are not translated, among them RNAs
present in saliva, by which means the virus can be trans-
called EBERs (EpsteinBarr early RNA) and BamAs (these
mitted to uninfected individuals. Lytic replication of virus
last are transcribed from a region of the genome found in
in the oropharyngeal epithelium persists indefinitely, as
a BamHI restriction fragment called the A fragment). Lat
persistently infected B cells continue to seed it, although
II cells express additional proteins called LMPs (latent
the extent of shedding of virus into the saliva declines
membrane protein). Lat III cells express still more proteins
with time.
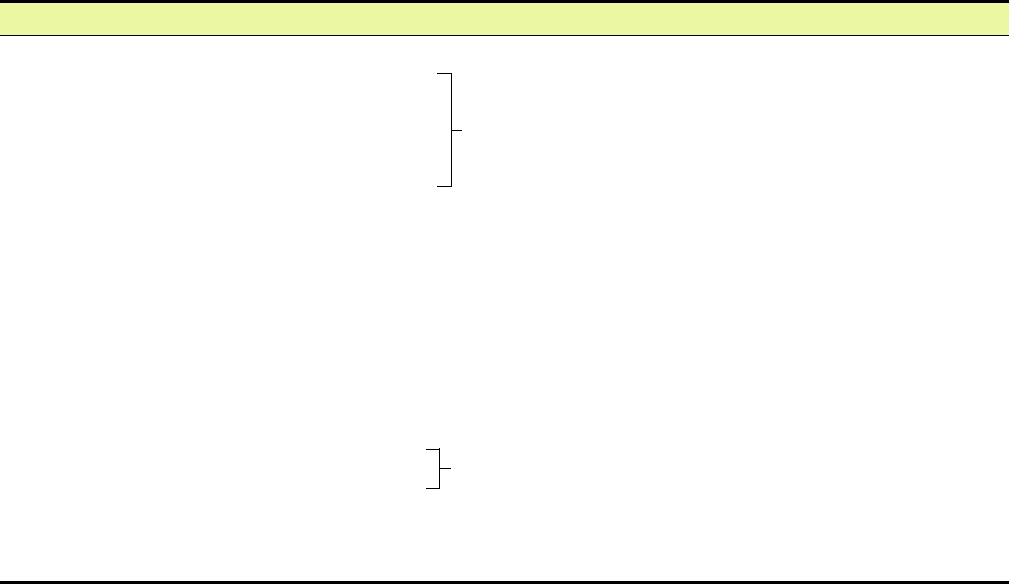
Stage of Latency
Transcripts
Proteins Expressed
Biological
Model
Consequences
EBNA1
EBNA 1 RNA
I
Bam A RNAs
EBV positive BL
EBERs 1,2
cells
Epstein-Barr
No cell division,
infection of
EBNA1
II
No CTL response
NPC cells
EBNA 1 RNA
B cells
LMP RNAs
LMP1, LMP2A,
LMP2B
Bam A RNAs
In vitro transformed
EBERs 1,2
LCLs
miRNAs
LMP1, LMP2A,
LMP RNAs
III
LMP2B
Proliferation
Spliced EBNA
EBNAs 1,2,3A,
Transformation
3B, 3C, and LP
transcripts
CTL responses
Bam A RNAs
EBERs 1,2
miRNAs
LCLs
Lymphoblastoid cell lines
Translated RNAs
EBNA-1 RNA
Burkitt s lymphoma cell lines
BL
Nontranslated RNAs
Bam A RNAs
NPC
Nasopharyngeal carcinoma cell lines
FIGURE 7.19 Stages of latency after EpsteinBarr infection. Latency I was first described in BL cell lines, but can
be reproduced by fusion of in vitro transformed LCLs with EBV-negative hemopoietic cell lines. Similarly, latency II
was first described in NCP cells but can be reproduced by fusing LCLs with certain human epithelial, fibroblast, or
hemopoietic lines. Data from Fields et al. (1996), pp. 23992402.
called EBNAs 2, 3A, 3B, 3C, and LP. One model for the
production of progeny virus in cultured cells. Replication
functions of these different types of latency is that Lat III is
of EBV under these conditions appears to involve path-
first established and serves to amplify the pool of infected
ways similar to those of HSV, with which it shares many
B lymphocytes, since Lat III cells are stimulated to divide.
genes (Fig. 7.13B).
Lat III cells are targets of CTLs, however, which kill these
The control of latently infected B lymphocytes by CTLs
cells in an attempt to control the viral infection. In contrast,
is obviously to the advantage of the virus as well as of the
Lat I and II cells are resting cells. The more limited set of
host. If the initial unlimited proliferation of infected B cells
proteins produced does not stimulate B cells to proliferate
continued indefinitely, it would be lethal for the host. This
nor make the cells targets of CTLs, and it is these cells that
in fact happens in people whose immune system is compro-
maintain the latent infection in the host. In this model, dur-
mised, as described later.
ing the lifelong infection by the virus, Lat I and II cells may
sporadically become permissive for lytic growth and pro-
Burkitt's Lymphoma
duce virus, or may sporadically switch to the Lat III state,
which results in cell division and the stimulation of CTLs
The ability of EBV to latently infect B cells and stimu-
that control these cells. Colonization of B cells is limited,
late continuous cell division leads to an association with
and it is estimated that only about one in 105 B cells is
a number of human cancers (Table 7.9). The first to be
infected by virus in asymptomatic carriers.
described was Burkitt's lymphoma (BL) by Denis Burkitt
The RNAs and proteins expressed in latently infected
in the 1960s in Africa. He attributed the disease to viral
cells are important for maintaining the latent state and induc-
infection and it is now known to be associated with EBV.
ing cellular proliferation. EBV DNA has three high-affin-
BL is a childhood malignancy that is worldwide but occurs
ity binding sites for EBNA1 and this protein enables the
predominantly in regions of Africa and New Guinea with a
circular EBV DNA to be maintained as an episome in the
high incidence of malaria. The tumors arise in lymph nodes,
infected B cells. LMP1 is an integral membrane protein with
frequently in submandibular nodes. The disease is fatal if
six membrane-spanning domains. It has transforming activ-
not treated, but treatment with chemotherapeutic agents
ity when expressed in certain rodent cell lines and is pre-
is effective and the majority of patients can be cured. The
sumably important for stimulation of B-cell division. It also
association with malaria was originally proposed to result
induces the production of cellular bcl-2, which protects the
from the suppression of CTLs induced by the microbe. Such
infected B cells from undergoing apoptosis. LMP2 is also an
suppression could result in an enlarged pool of EBV-trans-
integral membrane protein. It appears to prevent complete
formed cells, which are the progenitor cells that become
activation of the B cell so that the infection remains latent.
malignant. However, a more recent hypothesis proposes
EBNA LP, 2, 3A, and 3C are required for continuing growth
that it is the expansion of germinal centers that occurs
of the infected B cell.
in malaria that is responsible for the increased incidence
The EBER RNAs are abundantly produced in infected
of lymphomas. Germinal centers serve as sites in which
cells. They are small, nonpolyadenylated RNAs that are
somatic mutation occurs in the V gene of an antibody in
located mainly in the nucleus. They appear to be similar
order to increase the affinity of the antibody for its cog-
to the VA RNAs of adenoviruses described later. The vari-
nate antigen (see Chapter 10). BL cells are characterized
ous small RNAs produced upon EBV infection appear to
by deregulated expression of the cellular oncogene c-myc,
be involved in countering host antiviral defenses and in
and the c-myc expressed consistently carries mutations.
regulating aspects of cellular metabolism, functions that
Chromosomal translocations have been found in all BL
are important for the maintenance of latency and for the
tumors that result in placing the c-myc oncogene upstream
maintenance of lifelong persistent infection. For example,
of the immunoglobulin (Ig) genes. Ig genes are expressed in
microRNAs produced by EBV may target regulators of
B cells to high levels, and the translocation of c-myc to near
cell proliferation and apoptosis, chemokines and cytokines,
the Ig locus leads to its deregulated expression in B cells.
transcriptional regulators, and components of signal trans-
Perhaps its association with the Ig locus also leads to hyper-
duction pathways. The topic of microRNAs is covered in
mutation of c-myc and the development of a mutant form of
Chapter 10.
the gene that results in the development of a tumor. In any
Latently infected B cells sporadically become permis-
event, it appears that overexpression of a mutated c-myc is
sive for virus replication, perhaps as a result of differ-
essential for the development of BL.
entiating into plasma cells. In the infected human, this
Changes in genes other than c-myc also appear to be
results in production of virus and continued seeding of
required for development of BL. The cellular tumor sup-
the oral mucosa. In the laboratory, treatments of latently
pressor gene p53 is often altered in BL, and changes in other
infected B lymphocytes have been developed that result
cellular oncogenes or in chromosome architecture occur
in the conversion of a substantial fraction of them to per-
in some cases. These alterations may be important for the
missivity. This has allowed studies of lytic infection and
development of the full malignant phenotype. In addition,
TABLE 7.9
Tumors Caused by EpsteinBarr Infection
Tumor/subtype
Latent period
EBV positivity (%)
EB antigens
World distribution
Burkitt's lymphoma
Endemic
38 years
100
Infection primarily of children
90
<15 years of age in regions endemic
for malaria (P. falciparum)
EBNA1
Sporadic
38 years
1585
AIDS-associated
38 years post HIV
3040
Nasopharyngeal carcinoma >30 years
100
EBNA 1, LMP1, LMP2
Mostly in SE Asia
Hodgkin's disease
Mixed cell
>30 year
8090
EBNA 1, LMP1, LMP2
Worldwide, but more common in
Western hemisphere
Nodular sclerosing
>10 years
30
T-cell lymphoma
Fatal IM
<6 months
?100
?
Chinese and Caucasians
Nasal
>30 years
100
EBNA 1, LMP1, LMP2
AILD pleomorphic
>30 years
?40
?
Immunoblastic lymphoma
Fatal IM
<6 months
100
EBNA 1,2,3A, 3B, 3C
Transplant-associated
<6 months after
100
transplant
Transplant-associated
>1 year
100
LMP1 and LMP2
AIDS-associated
510 years post HIV
7080
Post EBV infection if not otherwise noted. IM, infectious mononucleosis; AILD, angioimmunoblastic-lymphadenopathy-like.
Source: Adapted from Fields et al. (1996) Table 2, p. 133.
BL cells downregulate several functions that are required for
that other events must occur in order for infected cells to
recognition and lysis by CTLs. In these cells the expression of
become malignant, as is the case for BL.
class I major histocompatibility complex proteins (MHC) and
of the transporter proteins (TAPs) that are required to trans-
T-Cell Lymphomas
fer antigenic peptides across the endoplasmic reticulum (see
Chapter 10) are reduced, effectively downregulating the pres-
The primary target of EBV in humans is B cells. However,
entation of antigens to CTLs by class I MHC. BL cells also
EBV has also been associated with some T-cell lymphomas
reduce or eliminate expression of cofactors required for effi-
(Table 7.9). Nothing is known about the process by which
cient interaction with T lymphocytes. Thus, a BL cell resists
the virus infects T cells and causes tumors.
lysis by CTLs, which are active in immune surveillance.
Thus, the transition from an infected B cell to a malignant
Nasopharyngeal Carcinoma
cell that can form a fatal tumor in an immunologically com-
petent person is a multistep process. Establishment of a latent
EBV is also associated with nasopharyngeal carcinoma
infection in B lymphocytes by EBV is only the first step.
(NPC). This disease is worldwide but has a much higher inci-
Many other events must follow, most of which are rare.
dence in Southeast Asia, in Eskimos, and among some popu-
Hodgkin's disease is another form of malignant lym-
lations in northern and eastern Africa. The available evidence
phoma associated with EBV. The disease often strikes young
suggests that both genetic and environmental factors are impor-
adults (hockey fans will remember that Mario Lemieux, a
tant for the higher incidence in these populations. Studies have
star of the Pittsburgh Penguins, underwent treatment for
found that a particular MHC haplotype (Chapter 10) is correlated
Hodgkin's disease). There is a second peak in incidence
with the relative risk of developing NPC. However, dietary fac-
after age 45. The disease is worldwide, although more com-
tors are also important because immigrant Chinese in the United
mon in developed countries. It is estimated that perhaps
States have a lowered frequency of NPC than people in China,
50% of all Hodgkin's disease is due to EBV. It is assumed
although the rate is still higher than that in Caucasians.
NPC is a carcinoma rather than a lymphoma, arising in
Infection of Humans with CMV
epithelial cells of the nasopharynx. Little is known about
Transmission of HCMV requires close contact between
how the carcinoma arises, but it presumably requires a non-
a susceptible person and a person shedding virus. Virus
lytic infection by EBV in which transforming genes are
present in oropharyngeal secretions, breast milk, or other
expressed. As for other tumors, several transforming events
bodily secretions is probably responsible for transmission.
are probably required for the carcinoma to develop.
It can also be transmitted by blood transfusion. The virus
is ubiquitous, present in all human populations, and most
EBV Infection in People with Compromised
humans become infected as infants. In different popula-
Immune Systems
tions, 40100% of persons become infected before the age
of puberty. HCMV infections are usually asymptomatic, but
People who are immunodeficient because of infection
primary infection of adults can result in infectious mononu-
with HIV or are pharmacologically immunosuppressed
cleosis, and primary infection or reactivation of viral repli-
following organ transplant are at greatly increased risk
cation in neonates or in the immunocompromised can have
for the development of lymphomas caused by EBV, as
serious consequences.
shown in Table 7.9. These lymphomas may develop after
HCMV infects epithelial cells in many different tissues,
a very short latent period, less than 6 months. Of inter-
in contrast to its restricted host range in cultured cells.
est is the finding that AIDS patients develop two forms
Infection characteristically results in cell enlargement,
of lymphoma. The first arises early, while the immune
from which the virus gets its name, and the presence of
system is relatively intact, and is a form of Burkitt's lym-
intranuclear inclusions. Shedding of infectious virus may
phoma, having the same c-myc chromosomal transloca-
persist for an extended period of time following primary
tion as described earlier. BL may arise at higher frequency
infection, in fact, for years if infection is congenital or
in AIDS patients in comparison to people with normal
occurs very early in life. Following control of infection
immune systems because of expansion of the infected B-
by CTLs, HCMV becomes latent, as do all herpesviruses.
cell population, giving rise to an expanded pool of poten-
Latency is probably established in leukocytes. Infection
tial precursor cells. The second form arises late, when the
is lifelong and, as for other herpesviruses, reactivation of
immune system is highly compromised. The late form
viral infection can occur and result in renewed shedding
appears to result from failure of CTLs to control the EBV-
of virus.
infected B-cell population.
A fatal, infectious mononucleosis-like illness in young
males has been described that is X linked (i.e., the suscepti-
Infection in Populations at Risk for Disease
bility gene is carried on the X chromosome, of which males
have only one copy). The disease is apparently due to a
Congenital infection by HCMV can be very serious if the
defect in the immune system that allows EBV-infected B
infant is not protected by maternal antibodies. About 1% of
cells to proliferate out of control. The disease is fatal 75% of
infants born in the United States are infected in utero, either
the time, and death usually results from uncontrolled immu-
as the result of reactivation of a latent infection in a seroposi-
noblastic lymphoma.
tive mother or as the result of primary infection in a seron-
egative mother. In the case of mothers who are seropositive,
maternal antibodies against HCMV, which are protective
Cytomegalovirus (HHV-5)
against disease, are transferred to the fetus. Congenital
The cytomegaloviruses (CMVs) are betaherpesviruses
infection then occurs with a frequency of only 0.22% and
that, like all herpesviruses, are species specific in their natu-
symptomatic disease does not occur in the infected fetus.
ral host range. They replicate slowly in cultured cells and
However, primary HCMV infection during pregnancy of
have a restricted host range in the laboratory. Human CMV
women who were previously seronegative results in infec-
(HCMV) will infect cultured human skin or lung fibroblasts
tion of the fetus up to 50% of the time, and about 10% of
as well as some peripheral blood monocytes. It will also
infections result in symptomatic infection in the newborn.
infect chimpanzee cells. Lytic replication in cultured cells
Infection may be fatal or may result in long-term neuro-
resembles that of HSV. There is regulated transcription of
logical sequelae, which may include defects in hearing or
α, β, and γ genes, and many of the genes are shared with
vision, seizures, microcephaly, or lethargy. Up to 80% of
HSV (Fig. 7.13B). However, the CMV replication cycle
symptomatic infants suffer severe neurological problems,
differs from HSV in one important aspect. CMV infection
and neurological impairment may occur even in the absence
leads to the stimulation of host-cell DNA, RNA, and protein
of symptomatic infection. Hearing loss is the most common
synthesis throughout infection, whereas infection with HSV
neurological sequela and congenital HCMV infection is the
results in the immediate shutoff of host-cell macromolecular
most common cause of hearing loss in the United States
synthesis.
other than that caused by genetic factors.
Like many other herpesvirus infections, HCMV infec-
ically related to betaherpesviruses like CMV and are now
tion, whether primary or resulting from reactivation of
classified as betaherpesviruses, in the genus Roseolovirus.
latent infection, is extremely serious in patients with
HHV-6 occurs as two major types, called A and B. The
compromised immune systems. It is often the most com-
virus is probably transmitted by oral secretions. In one study,
mon infection following organ transplant and can result
90% of adults were reported to have infectious virus in their
in life-threatening systemic disease. It is also a major
saliva, although other studies have given lower numbers.
life-threatening disease in AIDS patients. Latent HCMV
About half of children infected by HHV-6 suffer a disease
is present in most humans and systemic spread occurs
called roseola infantum, exanthem subitum, or sixth dis-
when the CD4+ lymphocyte count falls to very low levels.
ease, a mild disease of childhood that is characterized by
Systemic disease affects virtually every organ in the body,
fever and rash lasting 35 days. In one study, acute infec-
but infection of the lungs, central nervous system, and the
tion with HHV-6 accounted for 20% of visits to emergency
gastrointestinal tract are the most common and most seri-
rooms for febrile illness in 6- to 8-month-old infants. More
ous. Infection of the lungs can lead to fatal pneumonitis.
severe symptoms or neurological complications occur but
Infection of the central nervous system commonly results
infrequently. Primary infection of adults is rare because
in retinitis, which develops in 20% of long-lived AIDS
most people are infected as infants, but symptoms are more
patients. Infection of almost any region of the gastrointes-
serious when it does occur. The virus establishes latency in
tinal tract can occur and result in severe ulcerations that
monocytes and macrophages and a persistent infection in
can lead to perforation of the gut.
salivary glands and respiratory secretions.
The serious nature of disease in the immunocompro-
As for other herpesviruses, primary infection or recur-
mised shows that HCMV is an invasive virus that will
rence of infection in immunosuppressed people or peo-
infect many organs if not controlled by a vigorous immune
ple with AIDS can be life threatening. HHV-6 was first
response. Disease in transplant patients and in patients with
described in 1986 because of its association with lym-
AIDS is exacerbated by the expression of genes in HCMV
phoproliferative disorders in AIDS patients. The virus
that interfere with many aspects of the immune response.
can also cause serious problems in immunosuppressed
In immunocompetent people, these immunity-defeating
populations, in particular in patients undergoing bone
mechanisms allow the virus to live in harmony with the
marrow, kidney, or liver transplants, where infection or
host, establishing a lifelong infection that is associated with
reactivation can result in bone marrow suppression, pneu-
little or no disease. However, in the immunocompromised,
monitis, encephalitis, hepatitis, fever, or rejection of the
the thwarting of an immune response that is at best weak
transplanted organ.
leads to uncontrolled virus growth and serious illness. The
Conflicting evidence has also suggested that HHV-6
mechanisms by which HCMV interferes with the immune
might play a role in multiple sclerosis (MS). This chronic
response are described in Chapter 10. They include the syn-
disease is characterized by inflammation and demyelina-
thesis of several proteins that block the presentation of anti-
tion of neurons and has long been thought to have a viral
gens to CTLs by class I MHC, of a protein that interferes
etiology. A number of different viruses have been sug-
with the interferon response, and of a homologue to cellular
gested to be implicated in MS and HHV-6 is now one of
interleukin-10, which suppresses inflammatory responses,
them. Whether HHV-6 is in fact involved in MS remains
among others.
to be seen.
HHV-7 was found during studies of HHV-6 in peripheral
T cells. At present there is no clear evidence for the involve-
ment of HHV-7 in human disease. Of possible clinical
Human Herpesviruses 6, 7, and 8
importance is the fact that HHV-7 may use the same recep-
tor to infect CD4+ T cells as does HIV, which may allow
Three newly described human herpesviruses have come
to light in the last 2 decades and have simply been given the
HHV-7 to be used as a vector to express anti-HIV genes in
sequential numbers HHV-6, -7, and -8. They all appear to be
the specific target population infected by HIV.
typical herpesviruses that establish latent infections world-
HHV-8 is the most recently described human herpesvi-
wide. These infections are normally accompanied by no dis-
rus. It establishes a latent infection in B lymphocytes and
ease or only mild disease symptoms. The silence of their
is classified as a gammaherpesvirus, genus Rhadinovirus. It
infections caused them to be overlooked until recently, when
has a prevalence of 5% in the United States and is sexually
the ability of HHV-6 and HHV-8 to cause disease in immu-
transmitted. It was discovered through its association with
nocompromised populations, especially in AIDS patients,
Kaposi's sarcoma, the most common tumor found in patients
led to their discovery.
with AIDS. Tumor cells are of endothelial origin and are
HHV-6 and -7 have a tropism for lymphocytes, especially
multifocal. AIDS patients are much more likely to develop
CD4+ T cells. On the basis of this tropism they were first con-
Kaposi's sarcoma than are immunosuppressed patients, and
sidered to be gammaherpesviruses. However, they are genet-
there must be a synergism between the infections of HIV
Search WWH :