TABLE 7.3 Single-Stranded DNA Viruses
Hosta
Family
Genera
Genome size (kb)
Type species
Inoviridae
4.48.5
Inovirus
Enterobacteriaphage M13
Bacteria
Plectrovirus
Acholeplasma phage MV-L51
Mycoplasma
Microviridae
~4.46.0
Enterobacteria phage ΦΧ174
Microvirus
Bacteria
Spiromicrovirus
Spiroplasma phage 4
Spiroplasma
Bdellomicrovirus
Bdellovibrio phage MAC1
Bacteria
Chlamydiamicrovirus
Chlamydia phage 1
Bacteria
Geminiviridae
2.53.0
Mastrevirus
Maize streak
Plants
Curtovirus
Beet curly top
Plants
Begovirus
Bean golden mosaic-Puerto Rico
Plants
Topocuvirus
Tomato pseudo-curly top virus
Plants
Circoviridae
Circovirus
1.72.3
Porcine circovirus
Vertebrates
Gyrovirus
2.3
Chicken anemia
Vertebrates
[Unassigned genus]
Anellovirus
3.8
Torque teno
Vertebrates
[formerly Circoviridae]
Parvoviridae
4.06.0
Parvovirinae
See Table 7.16
Vertebrates
Densovirinae
Densovirus
Junonia coenia densovirus
Invertebrates
Iteravirus
Bombyx mori densovirus
Silkworms
Brevidensovirus
Aedes aegypti densovirus
Mosquitos
Vertebrates in red indicate humans are among the vertebrates infected. Vertebrates in blue indicate nonhuman hosts only.
a vigorous immune response. For such a strategy of long-
infect invertebrates (Table 7.1). Of the eight genera of the
term persistence to be successful, infection in the majority of
Chordopoxvirinae, seven contain viruses that infect mam-
hosts must be inapparent or cause only moderate symptoms
mals and one, Avipoxvirus, contains viruses that infect birds.
that are not unduly deleterious. Spread may be epidemic and
Only two human poxviruses (viruses for which humans are
accompanied by symptoms during primary infection, but for
the reservoir) are known, variola or smallpox virus, a mem-
some herpesviruses, vertical transmission to infant progeny
ber of the genus Orthopoxvirus, and molluscum contagio-
occurs without producing symptoms and persists for the life
sum virus, the only member of the genus Molluscipoxvirus.
of the animal. For such viruses, transmission needs to occur
The host range of any particular poxvirus is usually nar-
only once per generation for the virus to persist and a mini-
row. The two human poxviruses infect only humans in nature
mal population size is not required to maintain the virus in
and other poxviruses are similarly limited in their natural
nature.
host range. However, a number of mammalian poxviruses
whose primary host is not humans can cause natural, albeit
usually limited, infections of humans, and still other pox-
FAMILY POXVIRIDAE
viruses, including the avian poxviruses, can infect humans
under experimental conditions. Such mammalian and avian
The poxviruses are a very large family of dsDNA-con-
poxviruses have been used as agents for vaccination against
taining viruses that infect mammals, birds, and insects.
virulent human viruses (smallpox, described in detail later)
Eleven genera are recognized, eight of which are classi-
or as vectors to express foreign antigens for the purposes
fied as members of the subfamily Chordopoxvirinae and
of immunization (Chapter 11), because they normally cause
infect vertebrates (Table 7.4), and three of which are clas-
only a limited or an abortive infection of humans and can be
sified as members of the subfamily Entomopoxvirinae and
engineered to express foreign antigens.
TABLE 7.4 Poxviridae (Chordopoxvirinae: Poxviruses infecting vertebrates)
Genus/
Virus name
World
a
members
abbreviation
Usual host(s)
Transmission
Disease
distribution
Orthopoxvirus
Vaccinia
VACV
Unknown/humans, bovines
Contact
Localized lesions
Worldwide
Variola virus
VARV
Humans/none
Contact
Smallpox (now extinct)
Worldwide
Monkeypox
MPXV
Squirrels/humans, monkeys
Contact
Smallpox-like
West and Central
Africa
Cowpox
CPXV
Rodents/humans, cats,
Contact
Localized lesions
Europe, W. Asia
bovines, zoo animals
Camelpox
CMLV
Camels/none
Contact, aerosols
Localized lesions
Africa, Asia
Ectromelia
ECTV
Unknown/laboratory mouse
Contact, aerosols
Lesions plus
Europe
colonies, foxes, mink
disseminated disease
Volepox
VPXV
Voles/none
Contact
?
Western United States
Parapoxvirus
Orf
ORFV
Sheep/humans, ruminants
Contact
Localized lesions
Worldwide
Bovine papular stomatitis BPSV
Cattle/humans
Contact
Localized lesions
Worldwide
Pseudocowpox
PCPV
Cattle/humans
Contact
Localized lesions
Worldwide
Parapox of red deer
PVNZ
Red deer/none
Contact
?
New Zealand
Yatapoxvirus
MTBAb
Yaba monkey tumor
YMTV
Primates/human laboratory
Localized lesions
East and Central
infections
Many nodular
Africa
MTBAb
Tanapox
TANV
?Rodents/primates, humans
Molluscipoxvirus
Molluscum contagiosum MOCV
Humans/none
Contact, including
Worldwide
sexual transmission
lesions
Capripoxvirus
Sheeppox
SPPV
Sheep/none
Contact, fomites,
Asia, Africa
MTBA
Lumpy skin disease
LSDV
Cattle/none
Suipoxvirus
Swinepox
SWPV
Swine/none
MTBA, primarily
Generalized skin
Worldwide
by lice
disease
Leporipoxvirus
Myxoma
MYXV
Sylvilagus rabbits
MTBA, primarily
Benign tumors in
South America,
mosquitos
natural hosts, severe
Western United
disease in European
States, introduced
rabbits
into Australia
Rabbit fibroma
SFV
Sylvilagus rabbits
Eastern United
States
Benign tumors
MTBA
Hare fibroma
FIBV
European hare
Europe
in natural hosts
Squirrel fibroma
SQFV
Sciurus squirrels
Eastern and Western
United States
Avipoxvirus
Many species
Birds
Contact, MTBA
Lesions of skin and
Worldwide
including fowlpox
digestive tract
a
Hosts are listed as "reservoir host /other naturally infected hosts."
b
MTBA, mechanical transmission by arthropods.
The poxviruses are exceptional among eukaryotic DNA
Replication of Poxviruses
viruses because they replicate in the cytoplasm. The struc-
An overview of the poxvirus replication cycle is shown
ture of the virion is also unusual, being shaped like a brick
in Fig. 7.1. Following infection, an RNA polymerase within
rather than round or filamentous like most viruses. It has been
the core is activated by the release of the core into the cyto-
argued that the Poxviridae, Iridoviridae, Asfarviridae, and
plasm. This polymerase, together with accessory enzymes
Phycodnaviridae evolved from a common ancestor because
such as the capping enzyme and poly(A) polymerase,
they share a number of genes that distinguish them from other
synthesizes mRNAs that are capped and polyadenylated.
DNA viruses. The first three of these families contain viruses
These are extruded from the core and translated by the
of vertebrates that replicate in the cytoplasm, which as noted
host-cell machinery. Translation of early mRNAs leads to
is an unusual trait for DNA viruses, whereas the last family
further uncoating of the virus and the development of a
contains algal viruses that replicate in the nucleus.
regulatory pathway by which mid-cycle genes and finally
late genes are expressed. Early and mid-cycle functions
Structure of the Virion
include interference with host defense mechanisms and
the replication of the viral genome, whereas late genes are
Poxvirions are large and enveloped. They have a central
primarily involved with formation of progeny virions.
core containing the DNA complexed with multiple proteins.
Poxviruses have large genomes, from 130 to 380 kb, and
The core is biconcave in mammalian viruses (Figs. 2.1 and
encode hundreds of proteins. Because virus replication,
2.24). One or two lateral bodies (normally two in vertebrate
including DNA replication, occurs in the cytoplasm, pox-
viruses and one in invertebrate viruses) flank the core. These
viruses must encode all enzymes required for DNA replica-
contain proteins required for the initiation of viral replication.
tion and production of mRNAs. Thus, the encoded proteins
One or two viral envelopes, which contain a number of virus-
include DNA and RNA polymerases, a poly(A) polymer-
encoded glycoproteins, surround the core and lateral bodies.
ase to polyadenylate mRNAs, a capping enzyme, several
Altogether, virions contain 30 or more structural proteins.
enzymes with functions in nucleotide metabolism, protein
Vaccinia virus, which serves as a model for the family, is
kinases, DNA topoisomerases, as well as the proteins that
shaped like a brick with rounded edges and has dimensions
form components of the virion and the proteins that inter-
of approximately 360 × 270 × 250 nm. Two forms of infec-
fere with host defense mechanisms. Table 7.5 lists many
tious vaccinia virions are known, an intracellular form and
enzymatic proteins encoded by vaccinia virus and Table
an extracellular form. The intracellular form, referred to as
7.6 lists structural proteins and proteins without enzymatic
the intracellular mature virus or IMV, has a lipid-containing
activity. Poxvirus-encoded molecules that interfere with host
surface membrane and can be released by disruption of the
defenses are discussed in Chapter 10.
infected cell. The extracellular form, called the extracellu-
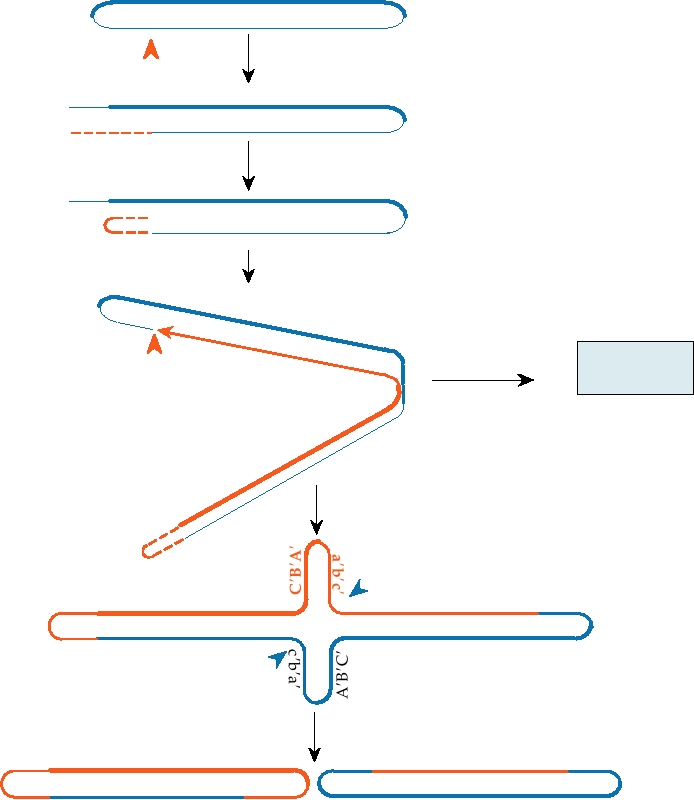
A schematic of the vaccinia virus genome is shown
lar enveloped virus or EEV, has a second, lipid-containing
in Fig. 7.2. It is double stranded and linear but the ends
envelope around it, which contains glycoproteins that are not
of the genome are covalently closed so that the genome
present in the intracellular form.
consists, in essence, of a very large single-stranded cir-
Vaccinia virus forms in viral factories in the cytoplasm
cular molecule that is self-complementary. The ends of
of the infected cell. A spherical immature virus 280 nm in
the genome possess inverted terminal repeats that are
diameter first forms in the cytoplasm whose surface mem-
involved in the initiation of DNA replication. A model
brane has been acquired from modified membranes called
for vaccinia DNA replication is shown in Fig. 7.3. The
crescents. Maturation of the immature virus to form the IMV
mechanisms by which DNA replication is initiated have
involves proteolytic cleavage by at least two viral proteases
not been completely worked out, but it is thought that a
that lead to the reorganization of the core and the lateral bod-
viral enzyme specifically nicks the DNA in or near the
ies and the formation of the brick-shaped structure. Some
terminal repetitions, and the 3′ end of the nicked DNA
IMV become further enveloped by a double membrane from
forms a primer for DNA synthesis. Continued elongation
the trans-Golgi or early tubular endosomes, the outer of
of the DNA chain leads to production of concatenated
which is lost as the virus exits the cell to become the EEV.
progeny DNA. These concatamers must subsequently be
Both IMV and EEV are infectious but attach to cells differ-
resolved into genome-length segments whose ends are
ently. The extra membrane in EEV helps to protect the virus
then covalently closed.
from immune surveillance.
Replication of DNA and assembly of progeny virions
The cellular receptors used by poxviruses to enter cells
occurs in what have been called viral factories in the cyto-
have not been characterized. The virus enters by fusion of
plasm. These are electron-dense areas that contain viral DNA
the IMV membrane with the cell plasma membrane. In the
and membranes. Progeny DNA is assembled into cores and
case of EEV, the outer membrane is first disrupted upon
assembly of virions occurs by condensation of membranes
binding to cellular receptors, allowing the IMV membrane to
around the viral core as described before.
fuse with the plasma membrane.
Infecting vaccinia virion
Growth factors,
2. Early mRNA
immune
1. Entry
defense
molecules
Transcription
5. Intermediate mRNA
(Steps 2, 5, and 6)
3. Uncoating
6. Late mRNA
4. DNA Replication
7. Translation
Late enzymes
Structural proteins
8. Assembly
9. Concatemer
NUCLEUS
resolution
10. DNA
Packaging
11. Maturation
12. Golgi wrapping
EUKARYOTIC HOST CELL
13. Exit
Virion-associated
enzymes
Progeny
RNA polymerase
vaccinia
Transcription factors
virions
Capping enzyme
Poly(A) polymerase
FIGURE 7.1
The replication cycle of vaccinia virus. Sequential steps are numbered in order. Note that despite the fact
that vaccinia is a DNA virus, the entire replication cycle takes place in the cytoplasm. Adapted from Fields et al. (1996)
p. 2638.
Interactions with the Host
factor), with the cytokine system so as to prevent an inflam-
Poxviruses produce proteins that stimulate cell develop-
matory response to the virus, or with the induction of apop-
ment and proliferation. Many poxviruses produce homo-
tosis (programmed cell death), among others. The discovery
logues of epidermal growth factor, which are excreted from
of proteins that interfere with host defenses is fairly recent,
the infected cell. Binding of this factor to its receptor induces
and intensive studies are ongoing to understand the full
the cell to enter pathways leading to proliferation and differ-
extent of viral inhibition of host defenses. The complexity
entiation. At least one poxvirus, orf virus, produces a homo-
of the interference by the virus with host antiviral defenses is
logue of vascular endothelial growth factor, which again
illustrated by the recent discovery that a viral protein called
induces cell proliferation upon binding to its receptor.
CrmB encoded by variola virus, which was known to be a
Poxviruses also produce a large number of proteins
homologue of the receptor for TNF and to inhibit the activ-
whose function is to interfere with the host immune system.
ity of TNF by binding to it, has a separate domain that binds
Vaccinia virus, variola virus, and other poxviruses encode
a number of chemokines and inhibits their activity as well.
proteins that interfere with the complement system, with the
Thus, this single protein inhibits a number of host defense
interferon system, with the activity of TNF (tumor necrosis
molecules. This discovery led to the further discovery that
TABLE 7.5 Vaccinia-Encoded Enzymes
Functional group
ORFa
name of enzyme
kD
Properties
DNA Replication
DNA polymerase
E9L
110
Protein kinase
B1R
34
Phosphorylates H5R
Unknown
D5R
90
Replication fork, ATP/GTP binding motif A
Uracil DNA glycosylase
D4R
25
Nicking-joining enzyme
??
50
Concatemer resolution
DNA toposiomerase
H6R
32
ssDNA binding protein
I3L
30
DNA ligase
A50R
63
Nonessential
DNA helicase
A18R
57
DNA-dependent ATPase
Early DNA-related metabolism
Thymidine kinase
J2R
20
Thymidylate kinase
A4w8R
23
Ribonucleotide reductase
Provide dNTPs
M1
I4L
87
Large subunit
M2
F4l
37
Small subunit
dUTP® dUMP, downregulate dUTP
dUTPase
F2L
15
DNA repair?
D9R
25
hydrolyze 8-oxo-GTP
D10R
29
NPH Ib
DNA dependent NTPase
D11L
72
RNA Transcription
RNA polymerase
Multisubunit enzyme
RPO147
J6R
147
RPO132
A24R
133
RPO35
A29L
35
RPO30
E4L
30
Transcription factor
RPO22
J4R
22
RPO19
A5R
19
RPO18
D7R
18
RNA polymerase-associated protein
H4L
94
RAP94, early promoter-specificity factor
Early transcription factor
DNA-dependent ATPase
A7L
82
ETR subunit 1
D6R
74
ETR subunit II
Poly(A) polymerase
E1L
55
Catalytic subunit
J3R
39
Stimulatory subunit, methyltransferase
Capping enzyme
RNA triphosphatase, guanyltransferase
D1R
97
Large subunit, catalytic activities
D12L
33
Small subunit, stimulates transferase
NPH IIb
RNA/DNA dependent NTPase
I8R
77
Protein kinase 2
F10L
52
Phosphorylates serines and threonines
Glutaredoxin
O2L
12
Thioltransferase, dehydroascorbate reductase
a
ORFs are named and color coded according to the restriction map shown in Fig. 7.2.
b
NPH, nucleoside triphosphate phosphohydrolase.
Source: Adapted from Fields et al. (1996) Table 3 on p. 2645 and data from Goebel et al. (1990).
TABLE 7.6 Vaccinia-Encoded
Inverted repeat
Inverted repeat
Unique sequences
Nonenzymatic Components
160,000 bp
10,000 bp
10,000 bp
Location in
ORFa
kD
Properties
virion
N K
P
J
Membrane of
I5L
8.7
Hydrophobic
M
O
L
intracellular
L1R
27.3
Myristylated, hydrophobic
C
F
E
I G
H
D
A
B
mature virus
*
H3L
37.5
Hydrophobic
Tandem repeats
Tandem repeats
13
18
18
13
H5R
22.3
Phosphorylated by B1R to
give 3436 kD
900 bp
1300 bp
1300 bp
900 bp
D8L
35.3
Cell-surface binding, virulence
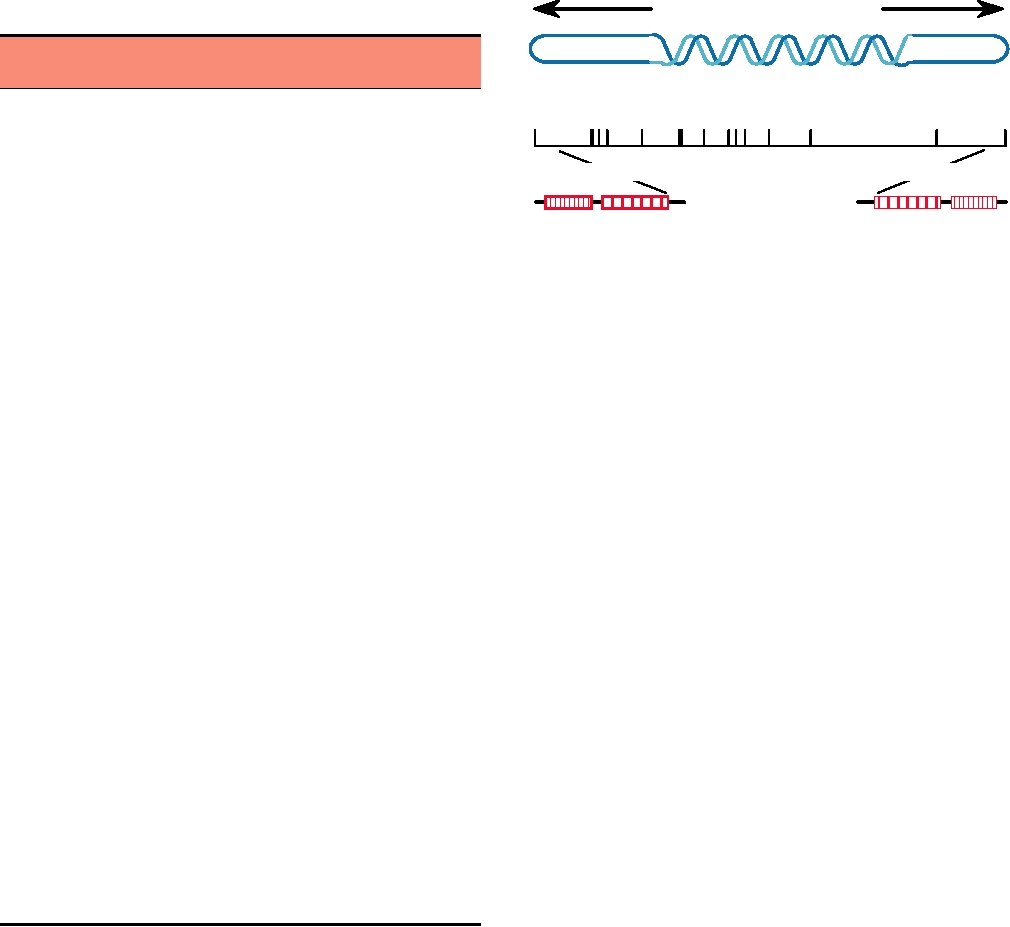
FIGURE 7.2 Schematic representation of the DNA of vaccinia virus.
D13L
61.9
Rifampicin resistance
Upper part: linear double-stranded DNA with terminal hairpins and inverted
A13L
7.7
Oligomeric
repeats (not to scale). Center line is the HindIII restriction map (* indicates
the TK gene). Color coding of the HindIII fragments is the same as that
A14L
10.0
Oligomeric
used in Tables 7.5 and 7.6. Lower line diagrams the internal structure of the
A17L
23.0
Dimer, neutralizing epitope
terminal repeats. Adapted from Fenner et al. (1988).
A27L
12.6
Fusion protein, neutralizing
epitope, required for EEV,
nonessential
Core of intracellular
F17R
11.3
Phosphoprotein, DNA
mature virus
binding
Genus Orthopoxvirus
I7L
49.0
Homology to toposiomerase II
The best known poxviruses are the orthopoxviruses.
G7L
41.9
Processed
Vaccinia virus has been widely studied in the laboratory as a
L4R
28.5
Structural protein VP8
model for the replication of poxviruses and has been used to
D2L
16.9
immunize hundreds of millions of people against smallpox
D3R
28.0
virus, also known as variola (from the Latin word for "spot-
A3L
72.6
Major core protein P4b
ted"). The extensive knowledge of vaccinia virus gained
A4L
30.8
from laboratory and clinical studies has also led to its use
A10L
102.3
Major core protein P4a
as a vector to express foreign antigens in cultured cells or in
animals (Chapter 11). Other members of the genus infect a
A12L
20.5
Processed
variety of domestic and wild animals (Table 7.4). Two simi-
Specific to enveloped
F13L
41.8
Envelope antigen
extracellular virus
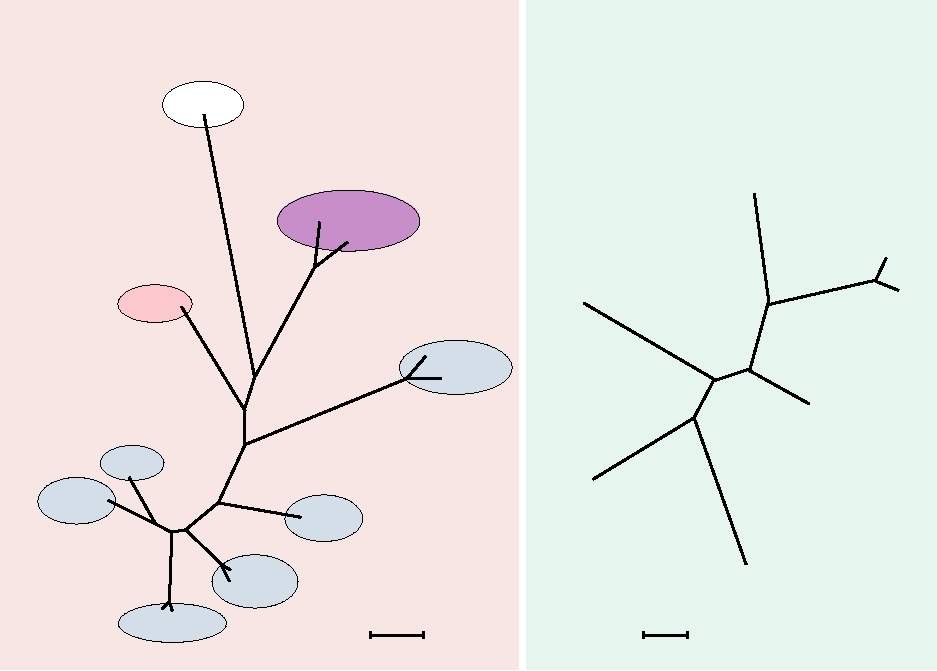
larity trees are shown in Fig. 7.4. One illustrates the rela-
A34R
19.5
N-glycosylated, homologous
(EEV)
tionships among the genera of the Chordopoxvirinae, and
to lectin, EEV release
the second illustrates the relationships among the orthopox-
A36R
25.1
viruses. All orthopoxviruses are sufficiently closely related
A56R
34.8
N- and O-glycosylated,
that they are cross protective.
hemagglutinin, nonessential
B5R
35.1
Complement control protein,
required for EEV,
Smallpox Disease
homologue to C3L
Smallpox once caused vast epidemics in human popula-
a
ORFs are named and color coded according to the restriction map shown
tions. It was already endemic in India 2000 years ago and had
in Fig. 7.2.
spread to China, Japan, Europe, and northern Africa by 700
Source: Adapted from Fields et al. (1996) Table 2 on p. 2643 with
A.D. It was introduced into the New World by the Europeans
additional information from Goebel et al. (1990).
during their explorations and settlement. Infection resulted in
a fatality rate of 2030% in most populations and at one time
the virus infected virtually the entire population of Europe.
Thus, the virus was responsible for a significant fraction of
all human deaths on the Continent. Data from London in
the 1700s show that, depending on the year, between 4 and
variola and other poxviruses produce a family of chemo-
18% of all deaths in the city were due to smallpox. When
kine inhibitors. It is clear that these various viral functions
introduced into virgin populations in the New World, the
are required for successful viral infection of their hosts in
mortality rate was much higher, probably due in part to a
nature, and the existence of these viral activities has been
lack of previous selection for resistance to smallpox and
very useful for our understanding of host defenses against
in part due to the breakdown of the social system caused
viral infection. We will return to this topic in Chapter 10.
ZC B A
ABCD
Z
zc b a
z
bc d
Nick of 3 strand
Primer extension
ZC B A
c b a ABCD
zc b a
CBAabc d
Loopback as primer
Z BA
C
c b a ABCD
ABC
cz a
b
abc d
Elongation through hairpin
BCD
CBA
abc
A
Nick at arrowhead to
Complex
start second round
Z cba
z
Concatemers
A
A
CB
a
a
cb
Simple concatemer forma-
tion
BC
d
c
ab
dcba
ABCD
Z
z
z
abc d
DAB C
Z
Resolve concatemers
ZC B A
abc z
dcba
ABCD
abcd
zc b a
ABCZ
DCBA
FIGURE 7.3 Model for the replication of orthopox DNA. Replication is initiated by nicking one strand at the red arrow
near the left end of the DNA. This is followed by primer extension and loopback to form an internal primer. Extension
then occurs through the hairpin at the right end of the molecule to form a concatemer. Concatemer resolution occurs by
nicking at the blue arrows. Parental DNA is shown in blue, new strands in red. Redrawn from data in Moyer and Graves
(1981) and Traktman (1990).
by the simultaneous infection of most of the population.
of the Americas by the Europeans was discussed in
As one example, smallpox coming up from Mexico swept
Chapter 4. A detailed description of the effect of smallpox
across the high plains of the western United States in the
virus on human civilization through the ages can be found
late 1700s, reducing the population of the Mandan, Ojibwa,
in the book Princes and Peasants: Smallpox in History by
Pawnee, Arikara, and other tribes by as much as two-thirds.
D.R. Hopkins. The history of nations has often been changed
Then in 1837 an epidemic of smallpox in Native American
by the early death of rulers from smallpox or from the appear-
populations along the upper Missouri River began with its
ance of smallpox in armies. As one example of interest to
introduction by passengers on a steamboat that came upriver
Americans, an invasion of Canada by American troops dur-
from St. Louis. In this epidemic it is estimated that 99% of
ing the Revolutionary War, with the idea of adding Canada
the Mandan died and half of the Hidatsa and Arikara. Other
to the American colonies, failed in part because an epidemic
tribes also suffered enormous losses leading to the depopu-
of smallpox swept through the American troops.
lation of the region. The importance of smallpox, measles,
Smallpox virus was exclusively a human virus, maintained
and other Old World plagues in the conquest and settlement
by person-to-person contact, and recovery from the disease
Chordopoxvirinae
Orthopoxviruses
A.
B.
Crocodilepox
CRV
Avipox
Camelpox
CNPV
FWPV
Variola major
Molluscipox
Monkeypox
Variola minor
MOCV
Parapox
ORFV
BPSV
Vaccinia
Suipox
SWPV
Capripox
Orthopox
Cowpox
SPPV
LSDV
VACV
ECTV
YLDV
Yatapox
Ectromelia
YMTV
0.02
0.2
SFV MYXV
changes per residue
changes per residue
Leporipox
FIGURE 7.4 Phylogenetic trees of the Chordopoxvirinae. (A) Unrooted tree illustrating the relationships between the
various genera. Eighty-three proteins from crocodilepox were aligned with similar data sets from other Chordopoxvirinae
using the program MUSCLE. Adapted from Afonso et al. (2006) Figure 5. (B) Unrooted tree of the Orthopox genus from
aligned coding sequences of the DNA polymerase gene. Adapted from Chen et al. (2003) Figure 3. Virus abbreviations:
CRV, crocodilepox; CNPV, canarypox; FWPV, fowlpox; ORFV, Orf; BPSV, bovine papular stomatitis; VACV, vaccinia;
ECTV, ectromelia; YLDV, Yaba-like disease; YMTV, Yaba monkey tumor; MYXV, myxoma; SFV, Shope fibroma;
LSDV, lumpy skin disease; SPPV, sheeppox; SWPV, swinepox; MOCV, molluscum contagiosum. Domains in (A) and
virus names in (B) are color coded by host, blue for vertebrates; pink and red for primates including humans; and purple
for birds. Scales indicate approximate number of changes per amino acid residue.
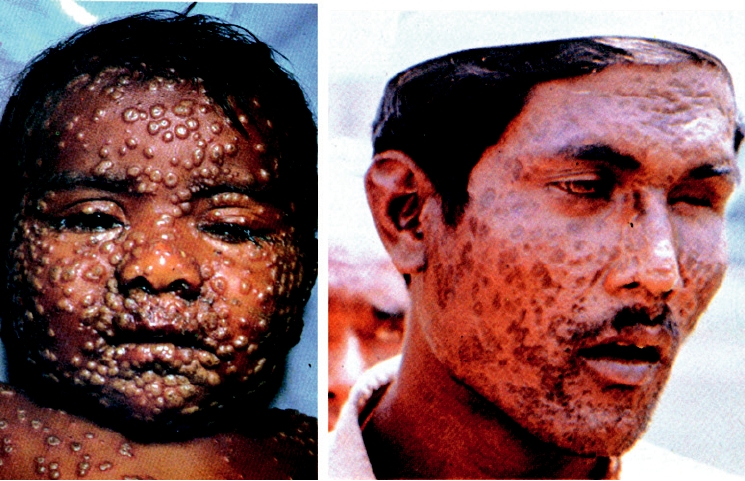
resulted in lifelong immunity to the virus. Thus, it was a dis-
7.5B is a photograph of a man who was blinded by smallpox
ease of civilization. As was described for measles virus, it
infection and illustrates the extent of facial scarring that can
must have originated from a nonhuman poxvirus, possibly a
occur as a result of smallpox.
poxvirus of a domesticated animal, at the time that the devel-
opment of human civilization led to the presence of large pop-
Immunization against Smallpox
ulation centers and the domestication of animals.
Although infection by smallpox begins as an upper res-
So great was the fear caused by smallpox that a technique
piratory disease, spread by aerosolization of virus shed from
of immunization was developed by the tenth century, called
skin poxes, infection becomes systemic and many organs are
variolation, in which people were deliberately infected by
infected. The virus replicates extensively in the skin, leading
smallpox using dried skin from pox lesions. The infecting
to pocks that can cover the entire body. These pocks leave
virus was delivered by blowing the inoculum up the nose
scars, particularly in the facial area, which mark a person for
or by inoculation into the skin. Infection via either method
life, and at one time the fear of scarring on recovery from
led to relatively low mortality, about 12% compared with
smallpox appears to have been at least as terrifying as the fear
2030% following natural infection, and the disease was
of death caused by the disease. A photograph of an infected
milder with less scarring.
child in Fig. 7.5A, taken on the eighth day of rash, illustrates
Jenner developed the modern concept of vaccination when
the ubiquitous nature of pox formation that can occur. Figure
he introduced and popularized the use of cowpox virus as a
A
B
FIGURE 7.5 Effects of smallpox. (A) Child with smallpox, on the eighth day of the rash. The rash began to develop
one day after the onset of fever. From Fenner et al. (1988) p. 15. (B) Adult after recovery, who is now blind and has deeply
pigmented pocks. From Fenner et al. (1988) p. 57.
vaccine against smallpox. It was known that milkmaids had
Vaccinia virus infection is localized to the area inoculated
beautiful complexions because they never contracted small-
in the vast majority of people, leading to a localized lesion
pox, and there was some evidence that their resistance to
that results in a pock. This vaccination procedure gave
smallpox resulted from infection with cowpox virus, which
solid immunity against smallpox but because of the limited
was occupationally acquired. In milkmaids, cowpox virus
replication of the virus in humans, the immunity was not
caused localized lesions, usually on the hands where they
considered to be lifelong and periodic reimmunization was
came in direct contact with the virus during milking, but the
practiced. Vaccinia virus has been used to immunize hun-
lesions did not spread. The virus is antigenically related to
dreds of millions of people over the years. It is generally
smallpox virus and will protect against infection with small-
safe, but infection of a small fraction of people results in
pox. Jenner's investigation in the 1790s included inoculation
a more serious disease. The most serious reaction in peo-
of a young boy with cowpox virus and then challenging him
ple with apparently normal immune systems is encephalitis,
with virulent smallpox virus, to which the boy was found
which is usually fatal but very rare (1 in a million vaccinees).
to be resistant, an experiment that could not be performed
Skin rash occurs more often but is not life threatening. In
today because of ethical considerations.
people whose immune system is impaired, progressive vac-
Jenner then introduced cowpox virus as a vaccine against
cinia may develop because the individual cannot control the
smallpox in the general population. This concept was contro-
replication of the virus, which results in a fatal illness.
versial at first, but gradually won widespread acceptance. The
virus now used for immunization is referred to as vaccinia,
Eradication of Smallpox
derived from the Latin name for cow. However, the relationship
between modern vaccinia virus and cowpox virus is a mystery.
Following the introduction of vaccination, the incidence
At some time in the last century, the source of the virus was
of smallpox declined and the virus was largely eliminated
changed and no one knows where vaccinia virus came from.
from developed countries. Interestingly, there also appeared
Modern vaccinia virus exhibits a fairly wide experimental host
a variant of smallpox that produced only a 1% mortality rate,
range. It has been suggested that it was derived from a domestic
called variola minor or alastrim (from the Portuguese word
animal other than the cow, perhaps a horse.
meaning something that "burns like tinder, scatters, and
Jenner "vaccinated" people by placing a drop of vaccinia-
spreads from place to place"). Variola minor was endemic in
containing solution on the skin and then scarifying the skin
Africa and the Americas and coexisted with variola major.
in some way, allowing the virus to replicate in this region.
Beginning in the 1960s, the World Health Organization
(WHO) began an intensive campaign to eradicate smallpox
the rationale being that smallpox virus represents a great
virus from human populations. Such a campaign was pos-
threat to the human population, which is now largely non-
sible because smallpox was exclusively a human virus, there
vaccinated and within another generation will be completely
was only a single serotype, vaccination with vaccinia virus
susceptible to infection by smallpox. If the virus were to be
was safe and effective, and inapparent infection was virtually
released, whether accidentally or deliberately, it could again
unknown. Every case of smallpox was tracked down, infected
cause devastating epidemics. However, the original date for
patients were quarantined, and all contacts were immunized
destruction of viral stocks has already passed and the dead-
against smallpox. Over a period of about 10 years, smallpox
line has been extended more than once. This issue has been
epidemics became less frequent and more completely con-
controversial because many scientists believe that further
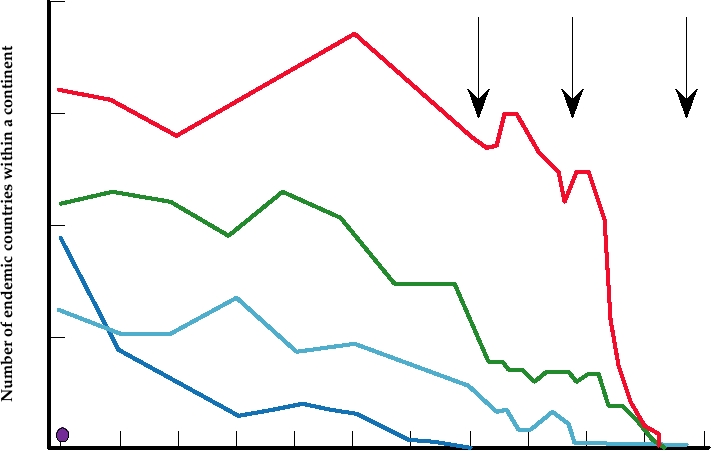
tained until finally the virus was, in fact, eliminated (Fig.
useful information can be obtained from the study of small-
7.6). The last case of natural smallpox occurred in 1977, and
pox virus. The genes that block human antiviral defenses
vaccination against the virus is no longer practiced because
have only recently been described, for example, and there are
of the possible side effects of the vaccine. Smallpox is the
surely secrets of viral virulence that we do not understand.
first virus to be deliberately exterminated, and the effort and
Furthermore, new forms of smallpox virus might arise. For
dedication required to accomplish this were remarkable.
example, monkeypox virus causes a disease in humans that
is similar to smallpox but with greatly reduced transmissi-
bility. Variants or recombinants able to spread more readily
What Do We Do Now?
could well arise. (Could this have been the origin of small-
The remaining, known stocks of smallpox virus are now
pox in the first place?) Thirdly, there is no guarantee that if
stored in two laboratories, the Centers for Disease Control
the known smallpox stocks in the United States or in Russia
and Prevention in Atlanta and the Vector Laboratories
were destroyed, smallpox virus would cease to exist. There
in Novosibirsk. These viruses are still being worked with
may be other stocks in laboratories around the world (the
and there is an effort being made to sequence many dif-
virus was very widespread at one time and extensively stud-
ferent strains of the virus and to obtain cDNA clones of
ied). It is known that the Russians weaponized smallpox at
them. The WHO has adopted the principle that all remain-
one time, and the virus would make a good agent for bioter-
ing stocks of smallpox should at some time be destroyed,
rorists, an all-too-real concern in today's world.
40
(1)
(2)
(3)
Africa
30
Asia
20
Europe
Americas
10
Oceania
0
1923
28
33
38
43
48
53
58
63
68
73
78
Year
FIGURE 7.6 Number of countries reporting the occurrence of endemic smallpox between 1923 and 1978, grouped by
continents. (1) marks the WHO resolution on global smallpox eradication in 1959; (2) shows the start of the intensified
eradication program in 1967; (3) marks the last case of smallpox on October 22, 1977 in Somalia. From Fenner (1983).
Because of concerns that smallpox might reappear, there
It infects humans under natural conditions but was thought
is a need to develop new vaccines against it. Although small-
to be only a rare zoonosis. The disease in humans caused
pox was eradicated using vaccinia virus as a vaccine, the
by monkeypox virus is clinically very similar to smallpox,
virus is more reactogenic than is tolerated in modern vac-
and it was not recognized as a distinct human pathogen until
cines, and the production of the vaccine uses outmoded tech-
the eradication of smallpox in Africa. There are two strains
nology. Further, people with an impaired immune system, an
of monkeypox virus, a West African strain found in Sierra
increasing fraction of the population, cannot be given vac-
Leone, Nigeria, Liberia, Ivory Coast, and Gambia and a
cinia virus. To develop a new vaccine that is safe and effec-
central African strain found in Zaire (now the Democratic
tive, an animal model is important. A model of smallpox
Republic of Congo) and the Central African Republic. The
disease in cynomolgus macaques has been developed, using
West African virus causes many fewer cases and the cases
infection by variola virus itself, another reason to keep stocks
are less severe with no deaths reported to date. The cen-
of the virus. Approaches to producing an effective vaccine
tral African strain has caused almost a thousand cases of
that is safer than vaccinia include using highly attenuated
severe disease since 1970 with a fatality rate of about 10%
virus. One approach is to use replication incompetent virus
in individuals who had not been vaccinated for smallpox.
as a possible vaccine. Replication incompetent viruses can
Epidemics of monkeypox in the Democratic Republic of
be derived from vaccinia virus by deleting essential genes,
Congo in 19961998 resulted in about 400 cases of disease.
and viruses such as canarypox virus are inherently replica-
The first wave of these epidemics lasted from February to
tion incompetent in mammals. Such vaccine candidates can
August 1996 and involved 89 cases of clinical disease with
now be tested in a monkey model for their effectiveness in
six deaths. A follow-up investigation of monkeypox in the
preventing disease following smallpox infection.
area in February 1997, which included a hut-by-hut search for
active cases in 12 villages, gave evidence that up to 73% of
monkeypox cases resulted from secondary human-to-human
Monkeypox Virus
transmission (Fig. 7.7). Furthermore, three of the deaths
Monkeypox virus was thought to be a virus of monkeys
were in children less than 3 years old and a large propor-
but is now known to be associated with squirrels and rodents.
tion of cases were in persons <15 years old. Thus, it appears
30
Secondary Cases
Primary Cases
20
10
0
Feb Mar Apr May June July Aug Sept Oct Nov Dec
Jan Feb
1996
1997
Month of rash
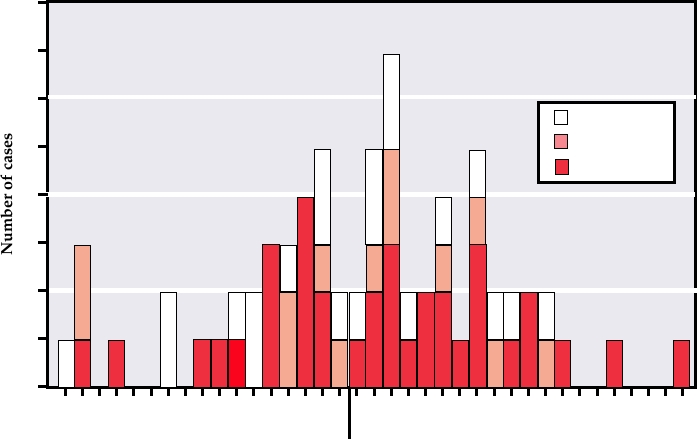
FIGURE 7.7 Number of monkeypox cases by date of rash onset in 12 villages in the Katako-Kombe health zone, Kasai-
Oriental, Zaire, February 1996February 1997. Note that most of the cases are secondary cases resulting from human-to-
human transmission. From MMWR (1997).
that the outbreak is the result of the cessation of vaccination
pox in this epidemic, 30 persons were immunized with the
against smallpox in the late 1970s, since smallpox vaccina-
smallpox vaccine. These vaccines included veterinarians,
tion is also protective against monkeypox. Monkeypox virus
health care workers, laboratory workers, and household con-
is therefore a human pathogen that can cause fatal illness,
tacts of patients. One vaccinee, who reported a rash, was
spread from person to person, and cause outbreaks of disease
confirmed as having monkeypox.
in susceptible populations. To date, spread has been limited,
but the potential for wider spread exits if the virus mutates so
Molluscum Contagiosum
that it is more readily transmissible from person to person.
Molluscum contagiosum virus is the sole representative
of the genus Molluscipoxvirus. It is a widely distributed
Monkeypox in the United States
human virus that is spread by contact, including sexual con-
tact. The virus causes a skin disease characterized by raised
An outbreak of monkeypox virus occurred in the
lesions. The disease is chronic but usually resolves within
Midwestern United States in 2003. The virus was imported
a few months, and the illness is considered to be trivial in
with a shipment from Ghana of more than 700 squirrels and
immunocompetent individuals. In HIV-infected patients,
rodents that included Gambian giant rats, rope squirrels,
however, the disease can be more troublesome. It has not
brushtail porcupines, tree squirrels, striped mice, and dor-
been possible to grow the virus in cultured cells, and all
mice. These animals, at least some of which were infected
virus for laboratory study has been derived from lesions of
with monkeypox, were intended as pets and were distributed
infected individuals, which usually contain large amounts of
to many states. One such transfer of some Gambian giant
virus. Despite this limitation, the genome of the virus has
rats and dormice went to a facility in Illinois where they
been completely sequenced and molecular biological studies
were housed in the vicinity of U.S. prairie dogs that were
are under way.
also intended as pets. The prairie dogs became infected by
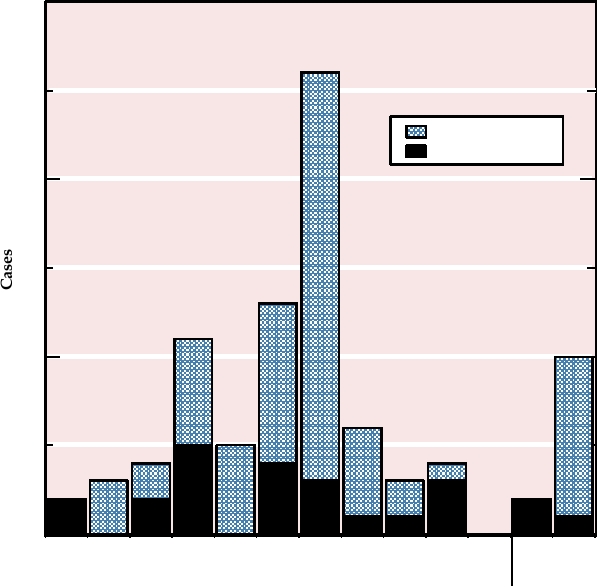
the virus and were subsequently distributed in seven states
including Illinois. A total of 71 people in six states became
Rabbit Myxoma Virus
ill with monkeypox contracted from the prairie dogs, 18 of
Rabbit myxoma virus, a member of the genus
whom were hospitalized. Half of the cases were confirmed
Leporipoxvirus, has been widely used in Australia to control
by laboratory testing (Fig. 7.8). There were no deaths from
populations of the European rabbit, which was introduced
this West African strain of monkeypox, which as described
with disastrous results into Australia by European settlers.
causes a milder illness in humans than does the Central
The history of the virus in Australia represents a facinating
African strain. To prevent the continuing spread of monkey-
8
7
6
Suspect
Probable
5
Confirmed
4
3
2
1
0
15
17
19
21
23 25
27
29
31
2
4
6
8
10
12
14
16
18
20
May
June
FIGURE 7.8
Number of monkeypox cases by date of illness onset during May and June of 2003 in Illinois, Indiana,
Kansas, Missouri, Ohio, and Wisconsin, reported as of July 8, 2003. Date of onset was unknown for two additional cases.
From MMWR (2003) Vol. 52, p. 642.
story of viral evolution in the field and is one of the best
in which people went out in large groups and killed as many
studied examples of coevolution of both a virus and its host.
rabbits as possible were undertaken in order to reduce the
The story has important implications for our understanding
rabbit population, but it was a losing game. To make matters
of virushost interactions.
worse, the introduced foxes preferred the Australian fauna
Rabbit myxoma virus is native to the Americas, where
to the introduced rabbits anyway, and constituted a second
it causes a localized skin fibroma in American rabbits. The
plague on the native fauna, which have not evolved to deal
virus is mechanically transmitted by mosquitoes. It does not
with mammalian predators.
replicate in the mosquito (it is not an arbovirus), but the mos-
In an effort to eliminate or control the rabbit population,
quito can transmit the virus when mouthparts become con-
rabbit myxoma virus was introduced. At first this strategy
taminated by feeding on an infected rabbit with skin lesions.
was very successful and the rabbit population was reduced
It was discovered early that, although the virus causes a triv-
by an estimated 95%. It was believed that the virus would
ial illness in American rabbits, it causes a systemic infection
have to be broadcast every year in order to continue the cam-
in European rabbits that is fatal more than 99% of the time,
paign against the rabbits, but there was hope that the rab-
a disease called myxomatosis.
bit plague would end. However, soon after its release, new
In Australia, a land of marsupials, the European rabbit
strains of virus arose that were less virulent, as illustrated in
was introduced for hunting or as potential food for foxes,
Fig. 7.9. These strains prevailed because they persisted more
which in turn had been introduced for the pleasures of fox
successfully in the rabbit population--less virulent viruses
hunting. The rabbits multiplied as rabbits proverbially do
remained within a host longer and were transmitted to new
and became a plague. The enormous populations of these
rabbits more successfully than virus that rapidly killed its
introduced rabbits depleted agricultural crops and also
host. Furthermore, rabbits that survived infection with the
threatened to overwhelm a number of Australian marsupials
less virulent strains were now immune to the virulent virus,
by competing with them for the food supply. A significant
making it more difficult to control the rabbits by reintroduc-
percentage of Australian native mammals have gone extinct
tion of a virulent strain (although rabbits have a short life
and rabbits have played an important role in the extinction of
span and herd immunity is not very important in resistance
a number of them. Rabbits have also caused problems from
to disease). With time the virus became progressively less
erosion by depleting large areas of ground cover. Campaigns
virulent but, interestingly, strains of very low virulence never
100
80
60
% Fatality MDOD
<13
>99%
Grade 1
95-99% 14-16
Grade 2
70-95% 17-28
Grade 3
50-70% 29-50
Grade 4
40
NA
<50%
Grade 5
20
0
1950
1954
1957
1960
1965
1968
1972
1978
Year
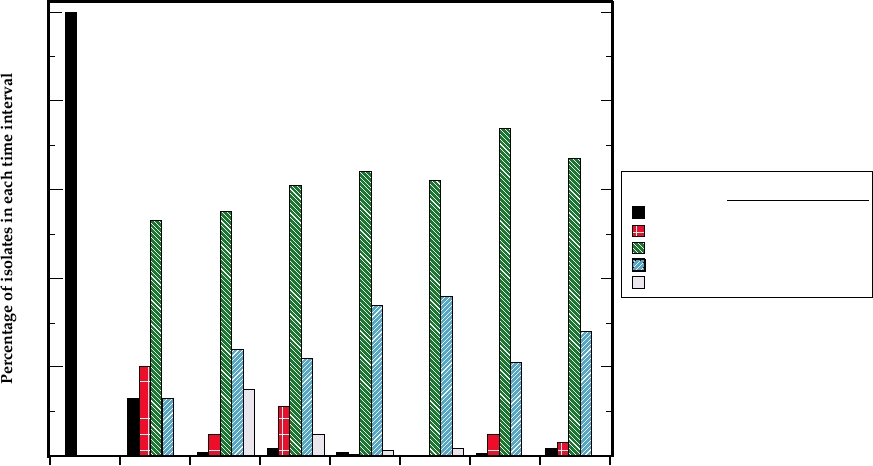
FIGURE 7.9 Virulence of field isolates of myxomatosis virus over a number of years in Australia after the introduction
of a grade 1 virus for biological control in 1950. Field isolates were classed as belonging to one of the five grades of
virulence on the basis of average survival times (MDOD = mean day of death) of six laboratory rabbits inoculated with
each isolate. This measure closely correlates with case fatality rates (which defined the original grades of virulence).
Isolates were collected over intervals of 3 to 5 years, and the collective data for each interval are plotted at a year in the
midpoint of the interval. Data from Fenner (1983).
Search WWH :