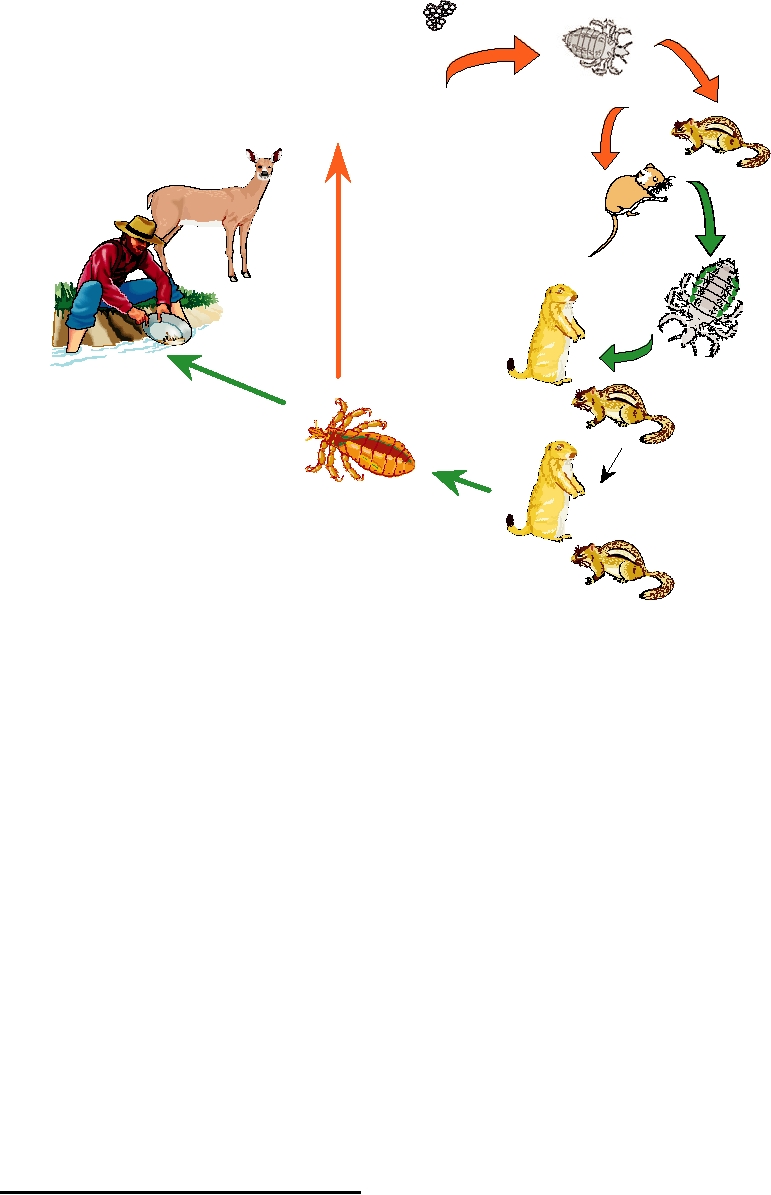
Eggs
Larval
Adult
Ticks
Ticks
Viremic Hosts
Nymphal Ticks
Uninfected Hosts
Adult
Ticks
Viremic Hosts
FIGURE 5.12 Natural transmission cycle of Colorado tick fever. Transfers of virus are shown with green arrows.
Larval ticks feed on small mammals that can remain viremic for long periods of time and then transmit virus to other
small mammals. Adult ticks, while not important for maintaining the virus in nature may then bite nonreservoir hosts
such as deer or man. Adult ticks lay eggs to produce the next generation of larval ticks, but no transovarial transmission
of virus occurs. Adapted from Bowen (1988) Figure 4.
particles suitable for genetic studies of the receptor-binding protein.
of an RNA synthetase that is packaged as part of the vir-
J. Virol. 75: 53355342.
ion. In contrast, packaging of the RNA synthetase in the
Joklik, W. K., and Roner, M. R. (1996). Molecular recognition in the
virion, which is necessary to begin the infection process,
assembly of the segmented reovirus genome. Prog. Nucl. Acid Res. 53:
and the retention of the genome in the entering subviral
249281.
Lee, P. W. K., and Gilmore, R. (1998). Reovirus cell attachment protein σ1:
particle, from which it is never released, are features that
Structure-function relationships and biogenesis. Curr. Top. Microbiol.
are shared with the (-)RNA viruses. Packaging of the
Immunol. 233: 137153.
RNA synthetase makes it feasible for a virus of verte-
Saragovi, H. U., Rebai, N., Roux, E., et al. (1998). Signal transduction and
brates to have its genome in multiple segments, a feature
antiproliferative function of the mammalian receptor for type 3 reovirus.
of reoviruses and of many (-)RNA viruses. Segmented
Curr. Top. Microbiol. Immunol. 233: 155166.
genomes allow reassortment to occur during mixed infec-
Schiff, L. A., Nibert, M. L., and Tyler, K. L. (2006). Orthoreoviruses
and their replication. Chapter 52 in: Fields Virology, Fifth Edition
tion, and reassortment is known to occur in nature. The
(D. M. Knipe and P. M. Howley, Eds. in chief), Philadelphia, Lippincott
acquisition of novel genome segments from related virus
Williams & Wilkins, pp. 18531916.
strains has occurred frequently during reovirus evolution
Shmulevitz, A., Yaameen, Z., Dawe, S., et al. (2002). Sequential partially
and has been advantageous for the survival of the virus
overlapping gene arrangement in the tricistronic S1 segments of avian
in nature.
reovirus and Nelson Bay reovirus: implications for translation initiation.
J. Virol. 76: 609618.
FUR THER READING
Rotaviruses
Reoviruses
Estes, M. K., and Kapikian, A. Z. (2006). Rotaviruses. Chapter 53 in: Fields
Chandran, K., Zhang, X., Olson, N. H., et al. (2001). Complete in vitro
Virology, Fifth Edition (D. M. Knipe and P. M. Howley, Eds. in chief),
assembly of the reovirus outer capsid produced highly infectious
Philadelphia, Lippincott Williams & Wilkins, pp. 19171974.
Glass, R. I., Parashar, U. D., Bresee, J. S., et al. (2006). Rotavirus vaccines:
Orbiviruses and Coltiviruses
current prospects and future challenges. Lancet 368: 323332.
Attoui, H., Jaafar, F. M., de Micco, P., and de Lamballerie, X. (2005).
Matthijnssens, J., Rahman, M., Martella, V., et al. (2006) Full genomic analy-
Coltiviruses and Seadornaviruses in North America, Europe and Asia.
sis of human rotavirus strain B4106 and lapine rotavirus strain 30/96 pro-
Emerg. Infect. Dis. 11: 16731679.
vides evidence for interspecies transmission. J. Virol. 80: 38013810.
Roy, P. (2006). Orbiviruses. Chapter 54 in: Fields Virology, Fifth Edition
Ramig, R. F. (2004). Pathogenesis of intestinal and systemic rotavirus infec-
(D. M. Knipe and P. M. Howley, Eds. in chief), Philadelphia, Lippincott
tions. J. Virol. 78: 1021310220.
Williams & Wilkins, pp. 19751998.
Sánchez-San Martín, C., López, T., Arias, C. F., and López, S. (2004).
Characterization of rotavirus cell entry. J. Virol. 78: 23102318.
Search WWH :