Borna disease virus is neurotropic and establishes a
behavioral abnormalities. Because of these effects on other
chronic or persistent infection despite an immune response
animals, several recent studies have tried to determine if the
to viral infection. The chronic infection results at least in part
virus is associated with neurological disease in man, in par-
because the virus downregulates its replication, resulting in
ticular with schizophrenia. Serological surveys have found
very low production of infectious virus. Downregulation to
that psychiatric patients are more likely to have antibodies
establish a persistent infection uses a mechanism different
to bornavirus than normal controls. Surveys which assay for
from those described in Chapter 3 for alphaviruses (shut-
the presence of viral RNA in peripheral blood mononuclear
off of minus-strand RNA) and for pestiviruses (titration of
cells (PMBCs) are even more suggestive: in some surverys
a cellular component required for RNA replication). Borna
up to 66% of psychiatric patients, including schizophrenics,
disease virus, like other (-)RNA viruses, has an inverted ter-
are positive for bornaviral RNA, compared to <5% of normal
minal repeat at the ends of the genomic RNA that contains
controls. Furthermore, very small amounts of virus-specific
promoters for RNA replication. During replication, the four
RNA have been isolated from postmortem brain samples
terminal nucleotides at the 5′ ends of both the genomic and
from patients suffering from schizophrenia and bipolar dis-
antigenomic RNA are often trimmed so that the majority
order, but not from normal individuals or patients suffering
of RNAs are missing these four nucleotides. The truncated
from other neurological disorders. Interestingly, a recent
RNA can be transcribed to produce mRNA but cannot repli-
study found that two patients hospitalized for severe depres-
cate, thus resulting in downregulation of RNA replication.
sion exhibited a rise in bornavirus antigen in PMBCs during
Borna disease virus appears to have a very wide host
the course of the disease, which fell to very low levels on
range. It was originally described as a pathogen of sheep and
recovery. Whether these different associations are indicative
horses in Germany, but is now known to infect a wide vari-
of causality remains to be determined, but it is conceivable
ety of warm-blooded vertebrates, birds as well as mammals.
that the virus causes recurrent episodes of depression on
The reservoir host in Switzerland has recently been reported
reactivation of a latent infection.
to be a shrew (Crocidura leucodon). These rodents are
exclusively terrestrial and it is thought that horses become
FAMILY OR THOMYXOVIRIDAE
infected by grazing on forage contaminated by excretions
from infected shrews.
As described, the virus establishes a chronic infection
The family Orthomyxoviridae (ortho = true or correct)
characterized by neurotropism and low production of virus.
contains three genera of influenza viruses: Influenzavirus A,
Infection may be asymptomatic or may result in disease
which contains influenza virus A; Influenzavirus B, which
characterized by movement and behavioral abnormalities.
contains influenza virus B; and Influenzavirus C, which con-
Naturally infected horses exhibiting such abnormalities usu-
tains influenza virus C (Table 4.7). Thogoto virus, a tick-
ally recover, but the disease may progress to paralysis and
borne virus of mammals, forms a fourth genus, Thogotovirus
death. Experimentally infected rats and primates also exhibit
and infectious salmon anemia virus belongs to a fifth genus,
TABLE 4.7 Orthomyxoviridae
Virus name
World
Genus/members
abbreviation
Usual host(s)
Transmission
Disease
distribution
Influenzavirus A
Influenza A
FLUAV
Humans, birds, swine
Airborne
Respiratory disease
Worldwide
Influenzavirus B
Influenza B
FLUBV
Humans
Airborne
Respiratory disease
Worldwide
Influenzavirus C
Influenza C
FLUCV
Humans
Airborne
Respiratory disease
Worldwide
Thogotovirus
Thogoto virus
THOV
Mammals
Tick-borne
Respiratory disease
Southern Europe,
Africa
Isavirus
Infectious salmon anemia
ISAV
Fish
Waterborne
Anemia, hemorrhagic
North Atlantic,
liver necrosis
North America
those of M (when present) and N of other (-)RNA viruses,
Isavirus. Influenza viruses A and B are closely related, but
respectively. The three proteins encoded in the three largest
influenza A infects a wide spectrum of birds and mammals
segments of influenza, called PB2, PB1, and PA (B or A
including humans, with birds being the reservoir, whereas
refers to a basic or acidic pK), possess the RNA polymerase
influenza B infects primarily humans and humans are the
activities encoded in the L protein and the P protein of other
reservoir. Influenza C is more divergent. Eight segments
(-)RNA viruses. Influenza A and B have two surface glyco-
of (-)RNA, totaling about 14 kb, comprise the genomes of
proteins, called HA and NA, but influenza C has only one,
influenza A and B viruses (Fig. 4.1) whereas influenza C
called HEF. These glycoproteins have the receptor-binding,
has only seven segments. Influenza viruses use sialic acid as
fusion, and receptor-destroying activities present in surface
a receptor, but the form used by influenza A and B viruses
glycoproteins of (-)RNA viruses.
differs from that used by influenza C virus, and the enzymes
Two proteins, called NS1 and NS2 (NS for nonstructural),
encoded by the viruses to destroy receptors are correspond-
are produced from RNA segment 8. NS1 is produced from the
ingly different. All three influenza viruses infect humans
unspliced mRNA (replication occurs in the nucleus). It binds
and cause disease, but influenza A represents the most seri-
to RNAs in the nucleus, including cellular pre-mRNAs, cel-
ous human pathogen because it causes very large, recurrent
lular snRNAs which are involved in splicing, and dsRNA.
epidemics with significant mortality. Influenza A has there-
Its activities inhibit the transport of cellular mRNAs from
fore been the most intensively studied and has been the focus
the nucleus and promote the synthesis of influenza mRNA.
of efforts to control influenza in humans.
NS1 also regulates splicing of influenza mRNAs and their
transport from the nucleus to the cytosol. Another function
Proteins Encoded by the Influenza Viruses
of NS1 is to interfere with the interferon pathway (Chapter
10), in part by binding dsRNA, which is a major inducer of
The proteins encoded in the different gene segments of
interferon and a cofactor for some proteins in the interferon
influenza A and influenza C viruses are described in Table
response to viruses, and in part by interacting with cellular
4.8. Influenza A produces 10 proteins from its eight genome
proteins involved in the interferon response. Influenza virus
segments, and most of these proteins have analogues in other
lacking NS1 is very sensitive to interferon and is replication
(-)RNA viruses (Fig. 4.1). The matrix protein, M1, and
defective in cells or hosts capable of synthesizing interferon,
the nucleocapsid protein, NP, perform functions similar to
TABLE 4.8
Genome Segments of Influenza Viruses
Influenza A
Influenza C
RNA
Encoded Protein
RNA
Encoded Protein
Functiona
segment
Length (nt)
Name
(aa)
segment
Length (nt)
Name
(aa)
1
2341
PB2
759
Cap recognition,
1
2365
PB2
774
RNA synthesis
2
2341
PB1
757
RNA synthesis
2
2363
PB1
754
3
2233
PA
716
RNA synthesis
3
2183
PA
709
4
2073
HA
566
Hemagglutinin, fusion,
4
2073
HEF
655
major surface
antigen, sialic acid
binding. HEF of
FLUCV also has
esterase activity
5
1565
NP
498
Nucleocapsid protein
5
1809
NP
565
6
1413
NA
454
Neuraminidase
7
1027
M1
252
Matrix protein
6
Spliced
M1
242
1180
p42
374
ßSignalase
M1′(p31) + CM2
See footnoteb
Spliced
M2
97
259 + 115
8
934
NS1
230
Nonstructural protein
7
934
NS1
286
See footnoteb
Spliced
NS2
121
Spliced
NS2
122
a
All functions other than those in footnote "b" apply to both influenza A and influenza C.
b
M2 of Flu A forms an ion channel, and NS2 of FluA is a nuclear export protein; the functions of the comparable moieties of Flu C are unknown.
Source: Adapted from Fields et al. (1996) Table 2 on p. 1355 and data in Fauquet et al. (2005) p 683.
this site. If the cleavage site consists of multiple basic residues
whereas the wild-type virus is resistant to the interferon
that can be recognized by the intracellular enzyme furin, the
pathway. NS2 is produced from a spliced mRNA. It inter-
virus can replicate systemically in at least some hosts. The SV-
acts with M1 attached to influenza RNP and promotes the
5 type 1 glycoprotein has only fusion activity and is called F.
transport of the RNP to the cytoplasm. It is present in small
As described before, it is produced as a precursor F0 which is
quantities in the virion and so is not truly nonstructural.
cleaved to F1 and F2, and the nature of the cleavage site affects
Protein M2 is produced from a spliced mRNA from seg-
the virulence of the virus (see Viral Glycoproteins under
ment 7. It forms ion channels in membranes, probably as a
tetramer, that allow passage of H+ ions. During transport of HA
Paramyxoviridae earlier in this chapter).
to the cell surface, the presence of M2 in the membrane of the
The receptor bound by both influenza A virus and by SV-
transport vesicle causes the pH within the vesicle to equilibrate
5 for entry into cells is sialic acid. The type 2 glycoprotein of
with that in the cytosol. This prevents low pH activation of
influenza has neuraminidase activity and is called the neu-
the fusion activity of HA during transport, because transport
raminidase or NA. It removes sialic acid from glycoproteins
vesicles are otherwise acidic. M2 is also present in virions and
for the same reasons as described for the paramyxoviruses
is required for the disassembly of the virus and for the activa-
that use sialic acid as a receptor. The type 2 glycoprotein of
tion of the RNA polymerase activity. To become active, the
SV-5 has both neuraminidase activity and receptor-binding
polymerase in the interior of the virus must be exposed to low
(hemagglutinating) activities and is called HN.
pH. Influenza virus enters the cell in endosomes, which are
Influenza HA is present as a trimer on the surface of the
progressively acidified. The acidic pH not only triggers a con-
virus (as is F of SV-5). The trimeric spike has a long stalk
formational change in HA that results in fusion of the viral
and a head containing the sialic acid binding sites. As shown
membrane with the endosomal membrane, but it also activates
in Fig. 1.6, exposure to acid pH in endosomes produces a
the RNA polymerase of the virion through the activity of M2.
dramatic rearrangement of the spike in which the fusion pep-
M2 is the target of the drug amantadine, one of the relatively
tide, which forms the N terminus of HA2, is moved over a
few drugs that are effective against a viral disease. Amantadine
distance of more than 10 nm to the tip of the spike. Here it
binds M2 of most influenza strains and prevents it from act-
inserts into the target membrane and promotes fusion of the
ing as an ion channel, which prevents the activation of the
viral membrane with the target membrane. NA is present as
polymerase. When taken early during infection, amantidine
a tetramer (as is HN of SV-5), and forms a spike that is dis-
ameliorates the symptoms of influenza. A worrisome trend is
tinguishable in the electron microscope from the HA spike.
the appearance of amantidine-resistant variants of influenza, in
There is only one surface glycoprotein in influenza C, the
particular the H5N1 strain referred to as "bird flu."
hemagglutinin-esterase-fusion protein (HEF). Influenza C
In most, but not all, influenza A viruses an 11th protein
virus has, therefore, one fewer gene segments than influenza
(PB1-F2) is made. This protein is translated from an alter-
A. HEF has receptor-binding (hemagglutination), fusion, and
native reading frame from the mRNA of PB1. It is present
receptor-destroying activities. The receptor is sialic acid, but
in mitochrondria in infected cells and may serve to regulate
the activity that destroys the receptor is an esterase activity.
apoptosis by the cell.
The esterase does not remove sialic acid from proteins as
does NA of influenza A. Instead it removes the 9-O-acetyl
group from 9-O-acetyl-N-acetylneuraminic acid, the recep-
Influenza Glycoproteins
tor used by influenza C, and the virus does not bind to the
Comparison of the glycoproteins of influenza A virus and
deacylated sialic acid.
the paramyxovirus SV-5 is of interest. In both influenza A
virus and SV-5, one of the glycoproteins is type 1 (N terminus
Replication of Influenza RNA and Synthesis
out) and one is type 2 (C terminus out). In both cases, the type
of mRNAs
1 glycoprotein is produced as a precursor that must be cleaved
to activate the fusion activity required for entry into cells. The
Synthesis of influenza virus RNAs occurs in the nucleus,
type 1 glycoprotein of influenza A has fusion and receptor-
rather than in the cytoplasm as for most RNA viruses. This
binding (hemagglutinating) activities and is called the hemag-
makes possible the differential splicing observed for two of
glutinin or HA. The precursor is called HA0 and the cleaved
the mRNAs. Following infection by the virus, the viral RNPs
products are called HA1 and HA2 (which remain covalently
are transported to the nucleus and mRNA synthesis begins.
linked by a disulfide bond after cleavage of the peptide bond)
During synthesis of mRNA, influenza engages in a process
(Fig. 1.6). Cleavage is required to activate the fusion activity
called "cap-snatching." Capped cellular pre-mRNAs present
in the nucleus are bound by NS1, and the 5¢-terminal 1013
of the virus and the nature of the cleavage site influences the
nucleotides, containing the 5¢ cap, are removed by PB2. This
virulence of the virus. If the cleavage site consists of a single
basic amino acid, cleavage is extracellular and influenza rep-
oligonucleotide is used to prime synthesis of mRNA from
lication is restricted to the respiratory tract, and in the case of
the influenza genome segments, as illustrated in Fig. 4.13.
birds the gut as well, where there are enzymes that can cleave
Once initiated, other aspects of mRNA synthesis resemble
59
39
G
vcRNA
ppp-AGC AAAGCAGG
CCUUGUUUCUACU
A
15-22 nt
Replication
39
59
UUUUUU
vRNA
HO- UCGCUUUCGUCC
GGAACAAAGAUGA
U
(genome)
mRNA synthesis
"Cap-snatching"
39
59
A G
7
m
mRNA
AAAAAAAAAAAAA(PolyA)
m GpppX Y
GC AAAGCAGG
G A
10-13 nt
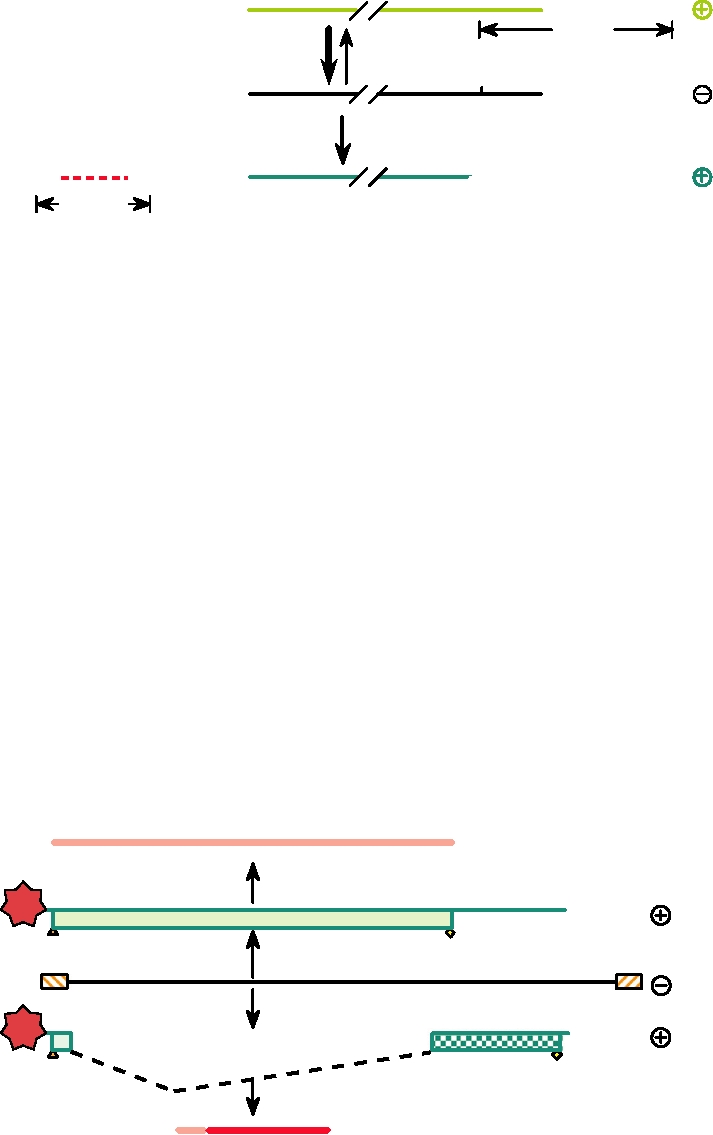
FIGURE 4.13 Relationship between genome RNAs, mRNAs, and vcRNAs of influenza virus. Transcription of
mRNAs in the cell nucleus requires a primer of 1013 nucleotides derived from cellular pre-mRNAs by "cap-snatching,"
and mRNAs terminate with a poly(A) tail. Those portions of the mRNA which are not complementary to the genome
RNA are shown in red. In contrast, vcRNAs are exact complements of the genomic minus strands. Adapted from Strauss
and Strauss (1997).
those that occur in rhabdo- and paramyxoviruses. Synthesis
mRNAs are formed from each of two of the segments, and
continues to near the end of the genome segment, where an
in total, 10 mRNAs are formed and 10 proteins are produced
oligo(U) stretch is encountered. Here the enzyme stutters to
(11 in the case of viruses that also produce PB1F2 described
produce a poly(A) tail on the messenger and then releases
earlier). The formation of the two mRNAs from segment 7
it. In addition to its role as a primer, using a cap derived
and their translation into proteins is illustrated schematically
from cellular mRNA relieves the virus of the necessity of
in Fig. 4.14.
encoding enzymes required for capping and ensures that the
When sufficient amounts of viral proteins have been
virus mRNA has a cap suitable for the cell in which it is
synthesized and transported to the nucleus, viral RNA rep-
replicating. This mechanism also results in interference with
lication begins. Replication requires encapsidation of prog-
the synthesis and transport of host mRNAs. Furthermore,
eny genomic and antigenomic RNAs as described for other
because the mRNAs have a different 5¢ end and lack the 3¢
(-)RNA viruses, and the mechanisms that lead to a switch
end of the antigenomic RNA, they lack promoters required
between synthesis of mRNAs and replication are thought
for replication and packaging and are therefore dedicated
to be similar to those that occur in rhabdoviruses and para-
mRNAs.
myxoviruses. During replication, the viral genome is cop-
Each genome segment gives rise to one primary mRNA
ied into a faithful antigenomic RNA (vcRNA) (Fig. 4.13),
species. However, two of these can be spliced, and both the
which is a perfect complement of the genome and serves as
unspliced and spliced RNAs serve as messengers. Thus, two
a template for production of genomic RNA.
M1 protein
(252aa)
Translation
59 CAP
39
M1 mRNA
Poly(A)
Cap-snatching, mRNA synthesis
39
59
Genome
Segment 7
Cap-snatching, mRNA synthesis,
59
39
splicing
CAP
M2 mRNA
Poly(A)
Translation
M2 protein
(97aa)
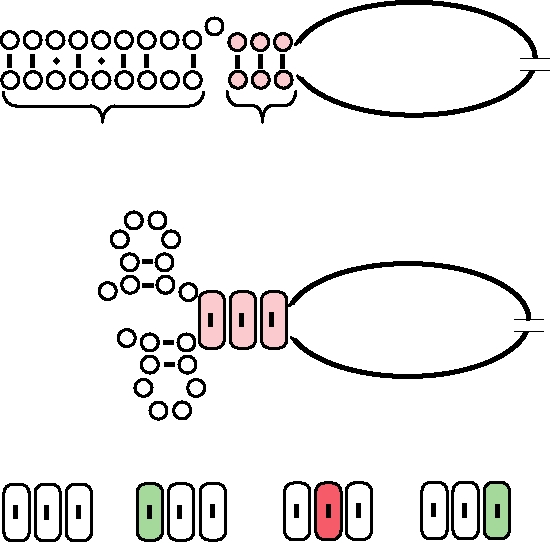
FIGURE 4.14 Synthesis of two mRNAs for the M1 and M2 proteins from gene segment 7 of influenza A. M1 RNA
is translated from ORF 1 (open box). M2 RNA starts identically, but after the splice it is translated in ORF2 (checkered
box). Both proteins are found in infected cells. The AUG initation codon is shown as a triangle; termination codons are
shown as filled diamonds. Patterned boxes at the end of the genome RNA are self-complementary sequences that could
form panhandles.
Synthesis of viral RNA, whether plus strand or minus
Assembly of Progeny Virions
strand, requires that the synthetase interact with both ends
of the RNA, whether vRNA or vcRNA; that is, the promoter
Influenza virus matures by budding of nucleocapsids
for synthesis of RNA is composed of elements from both
through the cell plasma membrane. Virions are pleomorphic
ends of the RNA. This is analogous to what has been found
but clinical specimens are primarily filamentous and can be
for alphaviruses and flaviviruses, described in Chapter 3,
up to a micrometer or more in length. Upon passage in cell
and may be a general mechanism used by many or all RNA
culture, most strains eventually give rise to virions that are
viruses. Thirteen nucleotides at the 5¢ end of the vRNA and
primarily spherical, averaging 100 nm in diameter. The form
12 nucleotides at the 3¢ end are highly conserved in influenza
that the virions assume is genetically determined. Studies of a
A viruses and these seem to contain the entire promoter ele-
strain of influenza A that remained filamentous after passage
ment. These sequences form an inverted terminal repeat and
in cell culture could be induced to form spherical particles
are capable of forming a panhandle structure (Fig 4.15A),
by changes in the M1 protein. The significance of filamen-
bringing the two ends together where they might interact
tous versus spherical particles is unknown, but filamentous
with the RNA synthetic machinery. An alternative structure,
forms must have a selective advantage in the infected ani-
called the corkscrew structure, is thought to be the structure
mal, whereas spherical forms seem to be selected upon pas-
recognized by the synthetase for initiation of RNA synthesis
sage in cell culture.
(Fig. 4.15B). Cyclization is also hypothesized to play a role
During assembly, the eight genome segments are reas-
in addition of poly(A) to mRNAs, by causing the polymerase
sorted in progeny virions if the cell is infected with more
to stutter at the oligo(U) tract located just before the double-
than one strain of influenza. Reassortment to produce
strand stem of the circular structure. Figure 4.15C illustrates
viruses with mixed genomes is efficient--the segments
an experiment to examine the sequence requirements within
are almost randomly reassorted to give all possible com-
the panhandle or corkscrew structure.
binations of genome segments in the progeny virions. This
A.
Panhandle Configuration
A
59
11 12 13
A G U A G A A A C
A G G
U C C
U C G U U U U C G
39
Region II
Region I
B.
Corkscrew Configuration (vRNA promoter)
G A
A
A
U A
59 A G C
11
12
13
A
G
G
A
39
C
G U
C
U C
G
C
U
U
U U
C.
Mutations in vRNA Promoter
11
12
13
11
12
13
11
12
13
11
12
13
A
G
G
A
G
A
U
G
G
A
G
G
U
U
C
A
C
C
U
C
C
U
C
C
Wt
Mutant D1
Mutant D2
Mutant D3
3 x 108 pfu
1 x 104 pfu
5 x 107 pfu
7 x 108 pfu
FIGURE 4.15 Models for the influenza A virus promoter. (A) The Panhandle model, with a partially double-stranded
structure for the 5′ and 3′ terminal sequences. (B) The Corkscrew model predicting base pairing within the ends. After
Neumann et al. (2004). (C) Alternative base pairs introduced into the vRNA promoter and the effect of these changes on
viral yield in MDBK cells. Adapted from Catchpole et al. (2003), Figure 1.
process is analogous to the reassortment of chromosomes
proteins do drift. This together with the fact that the viruses
that takes place during sexual reproduction in diploid
seldom cause disease in their avian reservoirs show that
organisms.
influenza in birds is ancient and the virus has adapted to its
Budding must result in the packaging of the 8 different
primary host.
genomic segments that constitute the viral genome into one
The gene segments of influenza A virus reassort read-
virus particle if it is to be infectious. Reoviruses (Chapter
ily during mixed infection, and viruses with new combina-
5) have an assembly mechanism whereby the 1012 differ-
tions of genes arise frequently. Newly arising reassortants
ent segments are recognized and assorted so that each virus
can cause major epidemics of influenza when introduced
particle has one each of the different segments. The case for
into humans, a process called antigenic shift. Not all com-
influenza virus is not completely clear. Evidence has been
binations of genes give rise to viruses that are capable of
presented that the virus appears to package more than 8 seg-
epidemic spread in humans. Only three subtypes of HA (H1,
ments, possibly about 10, that are randomly chosen from the
H2, and H3) and two or three subtypes of NA (N1, N2, and
intracellular pool. Random packaging of 10 segments would
possibly N8) have been found to date in epidemic strains of
result by chance in about 3% of the virions having at least
human influenza virus. The first influenza virus isolated, in
1 each of the 8 different genome segments. However, more
1933, was called H1N1. This virus first appeared as the cause
recent data argue that the virions package exactly 8 segments,
of the great influenza epidemic of 1918 (see later). The virus
one each of the 8 different segments. For this to occur, the
isolated in the epidemic of 1957 had a different subtype of
packaging machinery has to recognize internal sequences in
both HA and NA and was called H2N2. The H2N2 virus
each of the segments and not just a packaging signal in the
replaced the H1N1 virus as the cause of influenza epidemics
conserved ends of the viral RNAs.
(Fig. 4.17). The H2N2 virus was itself replaced by H3N2
virus beginning with the epidemic of 1968. Serological sur-
veys suggest that prior to 1918 the virus that circulated was
Influenza A Virus
an H3N8 virus that first appeared as the cause of an epi-
demic in 1890. The reason that only a subset of HAs appear
Natural History of Influenza Virus
to be capable of causing epidemics in humans is, at least in
part, the fact that the receptors for the virus are somewhat
Influenza A virus infects a wide variety of birds and mam-
different in birds and humans. Sialic acid is linked to galac-
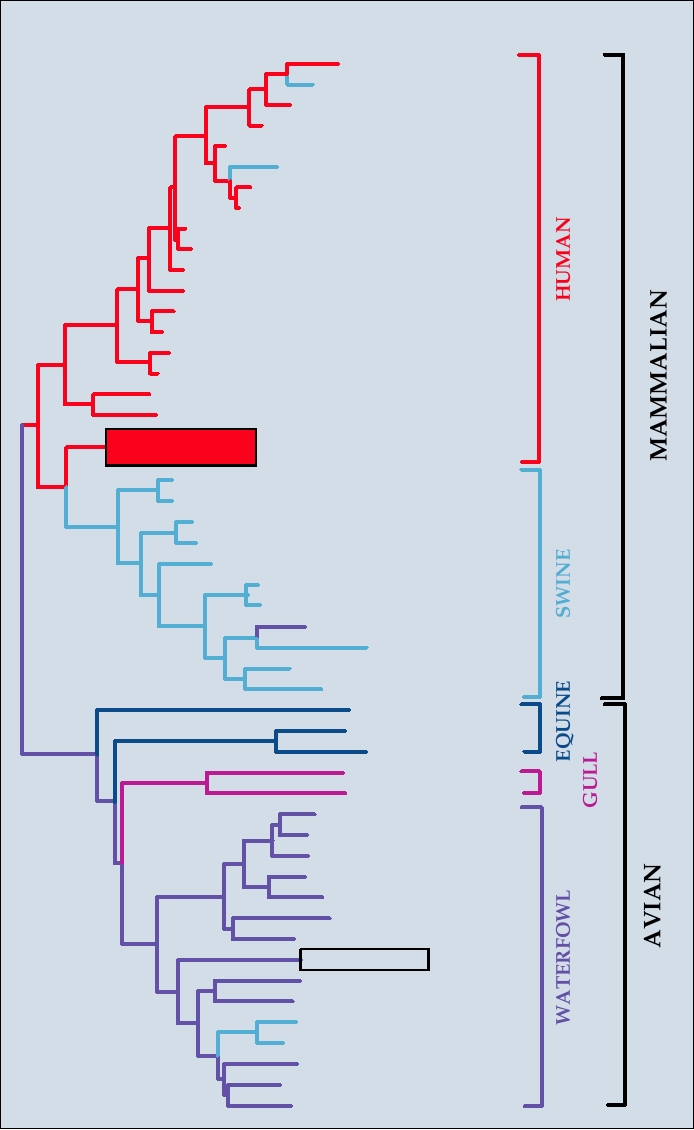
mals. A phylogenic tree that shows the relationships of the
tose predominantly by α2,6 linkages in humans but by α2,3
NP genes of viruses isolated from humans, pigs, and birds is
linkages in birds.
shown in Fig. 4.16. The human isolates and the pig isolates
Similarly, only certain types of the other segments
are closely related; as described later the pig viruses prob-
are compatible with infection of and epidemic spread in
ably originated from a human virus. The humanpig clade is
humans. For example, the nucleocapsid gene has diverged
distinct from the avian clade, however.
into five lineages, but only one of these lineages is present
Influenza A viruses are characterized by their two major
in viruses isolated from humans (see, e.g., Fig. 4.16). NS1
surface antigens, HA and NA. There are 16 different HA
is also at least partially host specific and thus only certain
subtypes (numbered H1 to H16). HAs in different subtypes
NS1s are compatible with human infection. Other proteins
differ by 30% in sequence and are not immunologically
also differ somewhat for optimal replication in birds versus
cross protective. There are also 9 different NA subtypes
mammals. It is thought that reassortment can result in the
(numbered N1 to N9). The major reservoirs of influenza A
introduction of a new HA or NA gene into a human virus,
in nature are wild ducks and other waterfowl such as gulls,
that is, a virus whose other gene segments are optimized
terns, and shearwaters, and viruses containing all 16 sub-
for human infection. The HA and NA proteins are the most
types of HA and all 9 subtypes of NA have been isolated
important antigens of the virus, and change of one or both
from waterfowl. Influenza replicates in the lung and in the
of these antigens gives rise to a virus for which the major-
gut of birds and the infection is normally asymptomatic (but
ity of the human population has no immunity and which is
epidemics of fatal influenza have occurred in turkeys and
therefore capable of causing a global pandemic. One pos-
chickens, and the emerging H5N1 virus has caused fatal
sible scenario is that pigs serve as intermediates ("mixing
infection in a number of different bird species). Ducks can
vessels") in the recombination process, because pigs can
excrete virus in feces for weeks, infecting other ducks via
be infected by both avian and human viruses (they contain
contaminated water, and a significant fraction of ducks may
sialic acid in both α2,3 and α2,6 linkage) and reassortment
become infected by the virus in this process. Migratory
could occur in this host.
ducks then spread the virus around the world, normally in
Influenza A virus is an example of a zoonotic disease in
a northsouth direction. The viruses in birds are in stasis.
humans. The reservoir of the virus is ducks and other birds,
Almost no differences in amino acid sequences of the vari-
and human infection is irrelevant for the maintenance of the
ous proteins are present in viruses separated by many de-
virus in nature.
cades, although the nucleic acid sequences encoding these
HOST
CLADE
SH90 (H3N2)
Sw/DN83(H3N2)
TE78(H3N2)
Udorn72(H3N2)
Vic86(H2N2)
Sw/HK76(H3N2)
Bei68(H3N2)
HK68(H3N2)
Sin57(H2N2)
MI60(H2N2)
Loy57(H1N1)
England55(H1N1)
Brazil78(H1N1)
FtWa50(H1N1)
FtMo47(H1N1)
Hickox40(H1N1)
WS33(H1N1)
PR34(H1N1)
1918 Influenza
Sw/IO46(H1N1)
Sw/IO30(H1N1)
Sw/OH35(H1N1)
Sw/37(H1N1)
Sw/May54 (H1N1)
Sw/WI57(H1N1)
Sw/WI61(H1N1)
Ty/NC88(H1N1)
Sw/NE98(H3N2)
Sw/TN77(H1N1)
Sw/HK82(H3N2)
Eq/Prague56(H7N7)
Eq/FL63(H3N8)
Eq/KY88(H3N8)
Gull/MD77(H13N6)
Gull/USSR84(H13N6)
Duck/PA69(H6N1 )
Duck/NY78(H2N2 )
Duck/TN76(H3N8)
Duck/Man.53(H10N7)
Ty/Ont.66(H5N9)
Ch/PA83(H5N2)
Ty/MN80(H4N2)
Gs/GD96(H5N1)
FPV34(H7N1)
Ch/Ger49(H10N7 )
Sw/Neth85(H1N1)
Sw/Ger81(H1N1)
Duck/Bav77(H1N1)
Tern/SA61(H5N3)
Duck/HK75(H3N2)
FIGURE 4.16
Phylogenetic tree of the nucleotide sequences of the influenza A virus NP gene sequences, constructed
with a neighbor-joining algorithm. Each isolate name includes the location and year of isolation, preceded for non-
human viruses by a species designation and a diagonal slash. Species abbreviations: Sw, swine; Ty, turkey; Ch, chicken;
Gs, goose. Standard two letter abbreviations for states in the United States are used. Other location abbreviations: SH,
Shanghai; DA, Dandong; Vic, Victoria; HK, Hong Kong; Sin, Singapore; Loy, Loygang; Ft Wa, Fort Warren; Ft Mo,
Fort Monmouth; Man, Manitoba; Ont, Ontario; GD, Guangdong; Ger, Germany; Neth, Netherlands; Bav, Bavaria; SA,
South Africa. The boxed isolate is the probable source of the H5 hemagglutinin in the currently worrisome "bird flu"
spreading from China. Adapted from Reid et al. (2004), Figure 2.
8
Type H2N2 appears
Type H3N2 appears
6
4
2
0
1935 1940 1945 1950 1955 1960 1965 1970 1975 1980 1985 1990 1995 2000
Year
Influenza A type H1N1
Influenza B
Influenza A type H2N2
Cocirculating B and A
Influenza A type H3N2
Cocirculating A types H1N1 and H3N2
FIGURE 4.17 Excess mortality caused by influenza A and B virus in the United States between 1934 and 1998. "1935"
refers to the winter of 19341935. Excess mortality due to the three dominant subtypes of influenza A and influenza B
are indicated by the colors shown in the key. Green cross-hatched bars are excess mortality in years when both A and B
viruses circulated. In 1955 and 1965, type H2N2 circulated with B, in 1983 and 1988 type H1N1 circulated with B, and
in 1985, 1992, 1996, and 1998 H3N2 circulated with B. In 1995 H1N1 and H3N2 types of influenza A both circulated
(hatched purple bar). Redrawn from Fields et al. (1996) p. 1421, with additional data from Thompson et al. (2003).
Epidemics of Influenza
Lower respiratory tract infection can also occur follow-
ing influenza infection and result in primary viral pneumo-
Influenza A virus causes a serious human illness, influ-
nia. Invasion of the damaged lungs by pathogenic bacteria
enza. It is perhaps confusing and unfortunate that the term
may follow and result in secondary bacterial pneumonia.
flu is often used to describe any respiratory tract infection
Influenza can be fatal, usually because of pneumonia result-
(and at times even infections of the gastrointestinal tract),
ing from viral infection, whether the pneumonia is due to
even those that are fairly mild. The symptoms of true influ-
primary viral infection or, more commonly, due to secondary
enza are usually more severe than those resulting from
bacterial infection. Fatal infection is more common in the
other respiratory tract infections and include fever, head-
very young (whose immune system is not fully developed)
ache, prostration, and significant muscle aches and pains
and in the elderly (whose immune system may be waning).
(myalgias) that last for 36 days. Weakness and cough can
Before the advent of antibiotics, bacterial pneumonia killed
last 12 weeks more. The fever can be high (3940°C is
many following severe bouts of influenza, but even today
not uncommon in adults and can be higher, especially in
influenza remains a serious killer. It has been estimated that
children). The morbidity that accompanies the disease can
influenza virus infects 1020% of the world's population
cause the patient to remain bedridden for a week or longer.
every year causing five million cases of severe illness and
In young children, the high fever can result in Reye's syn-
250,000 to 500,000 deaths. In the United States alone the
drome, an encephalopathy that may be fatal. The probabil-
estimated death rate from influenza in an average year is
ity of contracting Reye's syndrome is higher if aspirin is
20,00030,000 and can be significantly higher in epidemic
administered to control the fever.
years. People over 65 are at particular risk from influenza.
derly in 1917, the normal pattern, is apparent. The dramatic
The annual death rate in the United States from influenza A
increase in the death rate in the 20- to 29-year-old group in
in people over 65 is 1 per 2200, and in an epidemic year the
1918, in which people of this age were more likely to die
death rate may be 1 in 300 (i.e., 1 of every 300 people over the
than the old and the young, is striking. Death rates in young
age of 65 die of influenza during the epidemic). The excess
adults 1534 years of age were more than 20-fold higher
mortality caused by influenza is illustrated in Fig. 4.17, in
in the 19181919 pandemic than in the preceding years,
which the different strains of influenza A or B responsible
and the death toll in young adults in the United States was
for the epidemics are indicated. Although influenza A is usu-
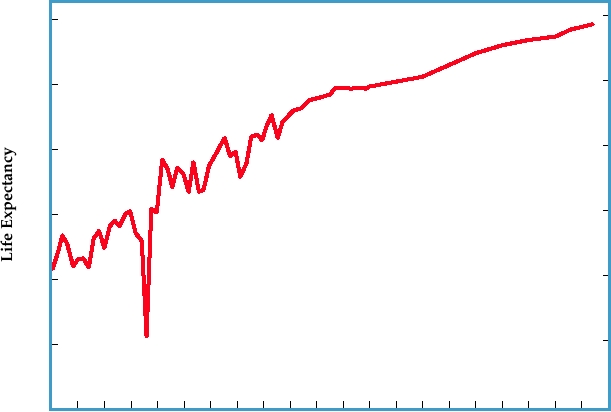
high enough that overall life expectancy dropped sharply, as
ally the most serious cause of mortality, in some years influ-
illustrated in Fig. 4.19.
enza B is more of a problem than influenza A.
The overall mortality was perhaps 2% of the world
population but in some regions of the world, for example,
regions of Central America and certain islands in the Pacific,
The 1918 Influenza Epidemic
1020% of the entire population died in the epidemic. In
A pandemic of influenza erupted in 1918 due to the emer-
some remote Alaskan villages, more than 70% of all adults
gence of a virulent H1N1 strain. This extremely virulent
died, usually as a result of the simultaneous incapacitation of
virus swept around the world over a period of about a year
the entire population so that supportive care was not avail-
and infected an estimated 30% of the world's population,
able. The final death toll can never be known with certainty
causing 20100 million deaths. Although the very young
and estimates vary widely, from 20 to 100 million. The death
and the elderly are normally at the most risk from influ-
toll exceeded that produced by World War I, which was
enza, this influenza pandemic of 19181919 was unusual
ongoing at the time. In fact, 80% of deaths in the U.S. Army
in that mortality was highest in healthy young adults. The
during World War I resulted from influenza, and it is thought
age distributions of people dying of influenza and the related
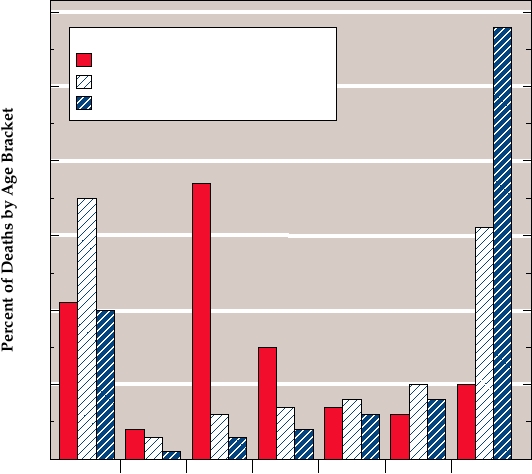
that the final collapse of the German army in 1918 may have
pneumonia are compared for the years 1917 and 1918 in Fig.
been precipitated by widespread influenza in the troops. The
4.18. The much higher death rates in the young and the el-
surgeon general of the United States had expressed the hope
60
Deaths due to:
Influenza and Pneumonia 1918
Pneumonia 1917
50
Influenza 1917
40
30
20
10
0
0-9
10-19
20-29
30-39
40-49
50-59
>60
Age Brackets (years)
FIGURE 4.18 Age distribution of deaths due to pneumonia and influenza in the United States in 1917 and 1918. Age at
death of patients has been divided into 7 intervals of 10 years each. The percent of deaths due to pneumonia in 1917, due to
influenza in 1917, and due to the combined effects of pneumonia and influenza during the great epidemic year 1918 which
fall into each age bracket are shown. The epidemic shows the atypical preponderance of deaths in the 2029 and 3039
year old brackets during the 1918 epidemic. Data from Crosby (1989). For comparison, from 1990 to 1998 only 3.8% of
deaths due to influenza and pneumonia occurred in persons <49, 4.75% in persons 5064, and 91% in persons over 65
years old (updated information from Thompson et al. 2003).
78
U. S. Life Expectancy
70
62
54
46
38
1918
1900
1910
1920
1930
1940
1950 1960
1970
1980
1990
2000
Year
FIGURE 4.19 Life expectancy in the United States, showing the precipitous drop in 1918 because of deaths due to
the "Spanish flu." This drop interrupted an otherwise fairly uniform increase in life expectancy that resulted from better
health care, sanitation, and living conditions. Note also the leveling off in the late 1980s and 1990s due to AIDS. Adapted
from ASM News, July 1999, and more recent data from the National Center for Health Statistics.
that WWI would be the first war in which more U.S. sol-
RNA in these tissue samples that could be used to reconstruct
diers died of war injuries than died of disease, but this hope
the complete sequences of genome segments. The sequences
was shattered by the influenza epidemic. Descriptions of
from these five victims are almost identical and showed that
the epidemic with a focus on its effects on U.S. society are
the virus belonged to strain H1N1. The HA genes from these
found in the books Flu, by G. Kolata, America's Forgotten
five humans differ by only one to three nucleotides despite
Pandemic, by A. W. Crosby, and, quite recently The Great
the fact that they came from five humans whose deaths were
Influenza, by John M. Barry.
separated by over 7500 miles and several months in time.
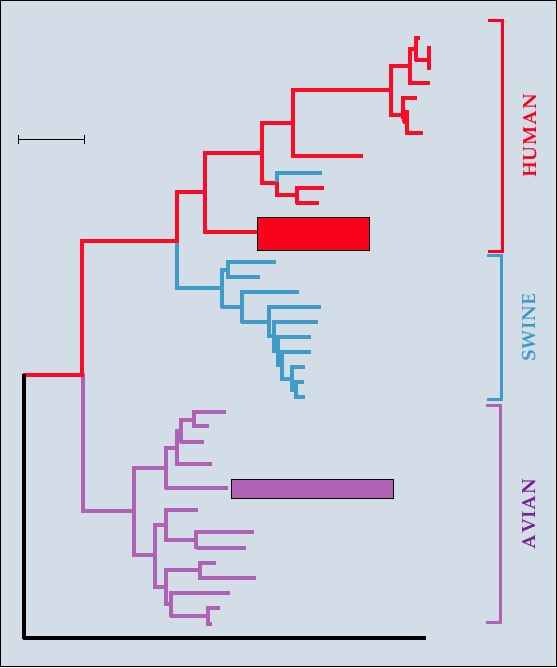
The reasons for the extreme virulence of the 1918 virus,
The sequence of this gene places it in the humanswine lin-
and why healthy young people were more likely to die, a topic
eage, not in the avian lineage, and at the root of the tree
made even more important by the appearance of H5N1 "bird
leading to later isolates of human or swine influenza (Fig.
flu" (see Chapter 8) have been addressed recently using the
4.20). Thus, the HA of the virus does not appear to have
power of modern molecular biology. The pandemic of 1918
come directly from an avian source.
occurred before influenza virus could be isolated. However,
It is now possible to use reverse genetics to take a cloned
the sequences of all eight gene segments of the 1918 influenza
DNA copy of an influenza gene and rescue a virus contain-
genes have been obtained starting from a number of tissue
ing this gene. To do this, cells are transfected with up to
isolates. Samples of preserved lung tissue taken at autopsy
17 plasmids that express the 8 genome segments of influ-
from two U.S. soldiers who died of influenza in September
enza as well as the RNA polymerase proteins PB1, PB2,
1918 in New York and South Carolina were found to con-
and PA, and the NP protein, and in some cases the other
tain detectable influenza RNA, albeit in fragmented condi-
influenza proteins as well. Infectious influenza virus is pro-
tion. A third source of influenza RNA came from an Alaskan
duced and buds from the cell. Using this system influenza
Inuit victim who died in November 1918 and was buried in
virus has been produced that contains various combinations
permafrost, and whose body was sufficiently well preserved
of the 1918 HA and NA genes with other cloned genes from
that lung samples containing (fragmented) viral RNA were
the 1918 virus or from recent isolates, including virus that
obtained. Two additional sources of influenza sequences
contains the complete complement of the 1918 genes and
come from two victims of influenza who died of pneumonia
thus is a complete reconstruction of the 1918 virus. Various
in November 1918 and February 1919 at the Royal London
constructs have been tested in mice. Whereas recent iso-
Hospital. Reverse transcriptasepolymerase chain reaction
lates of influenza virus cause only mild disease in mice, the
technology was used to obtain sequences from influenza
1918 virus causes severe, often fatal disease. In mice, a virus
Japan89
MA90
Stk90
Fiji88
USSR77
Len54
0.05
Brazil 78
Distance
PR34
Sw/Cambridge39
WS33
WSN33
1918 Influenza
Sw/IO30
Sw/29
Sw/IL63
Sw/NE92
Sw/Japan80
Sw/Quebec 91
Sw/IO88
Sw/NJ76
Sw/HK74
Sw/Italy81
Duck/Alb76
Duck/WI80b
Duck/WI80a
Duck/TN85
1917 Alaskan Brant
Gs/HK/76
Oy/Ger87
Duck/Aust/80
Ty/Ger91
Ty/Ger90
Duck/Bav77
Duck/HK76
Duck/HK77
H2 Japan57
FIGURE 4.20 Phylogeny of the H1 hemagglutinin genes (bases 494659 aligned to the comparable sequence of
PR34). Viral names include species of isolation followed by location and year of isolation. Species include: Sw, swine;
Gs, goose; Ty, turkey; Oy, oystercatcher. In the United States the standard two letter abbreviation for the state is used;
outside the United States the following abbreviations are used: Len, Leningrad; Ger, Germany; HK, Hong Kong; Bav,
Bavaria; Aust, Australia; Stk, Stockholm; Alb, Alberta. The sequence of the 1918 pandemic strain and the avian strain
most closely related chronologically are boxed. A distance bar, where a distance of 0.05 = 11.2 synonymous differences,
is shown above and beside the tree, and the H2 hemagglutinin of Japan 57 virus is used as an outgroup. Adapted from
Fanning et al. (2002).
containing only the H1 and N1 of the 1918 virus was found
immune system, and that healthy young people, who have
to be highly virulent and caused fatal infection in mice. Virus
the strongest immune systems, suffered from more extensive
grew to high titer in the lungs of the mice and was associ-
release of potent cytokines that resulted in more extensive
ated with an influx of neutrophils and macrophages into the
tissue destruction.
infected lung.
The devastation caused by the 1918 virus raises continu-
The complete 1918 virus has also been tested, under
ing concern that a strain of influenza of equal virulence might
BSL-4 high containment conditions, in monkeys as well as
appear and again cause immense suffering worldwide. New
mice. The virus caused severe, usually fatal, disease in mon-
pandemic strains of influenza appear three or four times a
keys that was marked by much higher replication rates and
century. If a pandemic strain emerged from a virus such as
more extensive spread in the lungs. It was also marked by an
the H5N1 strain of bird flu (see Chapter 10), which has a
abnormal innate immune response (see Chapter 10). Certain
very high mortality rate in humans, the resulting epidemic
elements of the innate response were attenuated, perhaps
could indeed be devastating.
because of the activity of the NS1 gene which is known
to interfere with the immune response. In contrast, other
Antigenic Shift and Drift
immune responses, in particular inflammatory cytokines,
were enhanced, resulting in a "cytokine storm." The results
Immunity to influenza A virus following infection is
are consistent with the hypothesis that in humans the 1918
long lived but may not be complete and is subtype specific
virus provoked an extreme but unbalanced reaction by the
and even strain specific. The continuing appearance of new
strains that arise from antigenic drift and of new subtypes
must be made by late spring in order to allow time for the
that arise from antigenic shift lead to continuing epidemics.
pharmaceutical companies to prepare the vaccines, and an
Normally, two or three strains of influenza A circulate in the
element of risk is involved that the right choices will not
human population at any one time. Spread from person to
be made. The World Health Organization publishes choices
person is by respiratory droplets, requiring close proximity,
and supplies seed virus based upon the recommendation of
but people travel extensively and new strains of the virus
an international group of scientists, but the final selections
speed around the globe as they arise. Antigenic drift is the
are made by individual health agencies and the choices are
process by which mutations accumulate in the virus genome,
usually, but not always, correct. The number of vaccine
usually because of immune selection, that result in the devel-
manufacturers has declined dramatically in the United States
opment of new strains of the virus. These new strains are
over the last 2 decades because of legal liability problems,
partially resistant to the immunity induced by infection with
and what limited capacity that exists for manufacturing flu
previous strains of virus. After several years of drift, the
vaccine is mostly present in Europe. Production problems
strain may be sufficiently distinct to cause disease in a per-
by one of the manufacturers has resulted in recent shortages
son previously infected, but the illness is usually less severe
of vaccine.
because of partial immunity to the new strain. However,
The necessity to grow the virus in fertilized eggs also
new strains capable of causing serious illness can arise by
limits the amount of vaccine that can be produced. There
antigenic shift whereby reassortment results in change of
are efforts to develop a cell culture system for virus pro-
the surface glycoproteins of the virus. The reassortants that
duction for vaccine use, which could then be produced in
cause the biggest problems are those belonging to a new
larger amounts. Efforts are also being made to develop bet-
subtype (as illustrated by Fig. 4.17). As described, such a
ter adjuvents for use with the vaccine, which could reduce
new subtype may cause a pandemic in the human population
the amount of antigen required per inoculation. In addition,
because there is little immunity to the virus carrying these
obtaining the reassortants required for vaccine production is
new surface antigens, as happened in 1918 (H1N1), 1957
a time-consuming endeavor using classical methods of coin-
(H2N2), and 1968 (H3N2).
fecting cells with two different viruses and searching through
H1N1 virus, which had disappeared with the appearance
the progeny for the wanted reassortants. If reverse genetics
of the H2N2 epidemic strain in 1957, suddenly reappeared
described earlier can be developed in a way that satisfies
in 1977. This H1N1 virus, which first appeared in northern
the regulatory agencies concerned with vaccine safety, the
China in May 1977 and was called the Russian flu, was vir-
desired reassortants could be obtained much more quickly,
tually identical to influenza virus isolated from an epidemic
allowing quicker responses to new strains of virus.
in humans in 1950. It circulated in young people who had
In addition to the inactivated virus vaccine that is very
not been exposed to H1N1 virus. Because it was virtually
widely used, a new live virus vaccine based on a cold attenu-
unchanged despite 27 years having elapsed, it seems unlikely
ated virus has been licensed recently. Reassortment is used to
that it arose again de novo. Presumably this virus had been
introduce the HA and NA of the predicted epidemic strains
preserved in a frozen state, probably in a laboratory freezer.
into this attenuated virus. Because the attenuation of the virus
In 1976, in response to reports that investigators outside
results from changes in other genome segments, the recom-
Western Europe planned to develop and test vaccines against
binant strain is also attenuated. The vaccine is administered
H1N1 influenza, a WHO meeting report urged extreme cau-
by nasal spray rather than by injection as is the inactivated
tion in developing live vaccines from epidemic H1N1 strains
virus vaccine. To date this vaccine is only licensed for use in
because of the possibility of spread of the virus. One year
people between the ages of 5 and 49, and thus it cannot be
later the virus reappeared.
used for the populations most at risk for serious illness, but
clinical trials are continuing. It remains to be seen how well
accepted this vaccine will be.
Vaccination against Influenza A Virus
The necessity of reformulating the vaccine every year
Because of the seriousness of influenza disease, especially
is inconvenient for a number of reasons including the fact
in the elderly, attempts are made each year to vaccinate the
that the vaccine cannot be stored for use in the follow-
population at risk. Because of drift and shift, the vaccine
ing years. In addition, the vaccine is not always effective
must be reformulated every year to reflect the viruses cur-
because wrong predictions were made about which strains
rently circulating in the human population. There are three
of virus would be the biggest problems. There is an effort
strains of virus included in the most common vaccine, an
being made to develop universal vaccines that would target
inactivated virus vaccine produced from viruses grown in
all strains of influenza A and B, and that would therefore
eggs. These are two influenza A viruses and one influenza B
provide protection against all influenza strains and that
virus. These viruses are chosen from those that are circulat-
could be used year after year. One possibility that is being
ing in late spring, because these viruses are usually those
pursued is to use influenza A M2 protein as an antigen.
that will cause epidemics the following winter. The choice
This protein is highly conserved among all A strains but is
not normally seen by the immune system for some reason.
pharmaceutical houses. The vaccine was never conclusively
Preliminary studies have shown that this protein linked to
shown to cause disease, although there seemed to be a slight
hepatitis B core protein is highly immunogenic in mice and
increase of Guillain-Barré syndrome following inoculation.
provides protection against influenza A infection in mice,
Litigation went on for years and substantial damages were
regardless of strain. For influenza B, a subunit vaccine
paid out. In retrospect it is easy to criticize the program as an
based upon the sequence surrounding the cleavage site of
overreaction, but what would have been the reaction if noth-
the HA precursor, which includes a highly immunogenic
ing had been done and an influenza epidemic developed that
part of the fusion peptide, shows promise in early animal
resulted in 50100 million Americans becoming seriously
trials.
ill with 12 million deaths? Given the state of knowledge
at the time, many leaders felt there was no choice. Further,
the decision to vaccinate was not so different from cur-
Swine Flu Virus
rent policy, where strains of influenza A circulating in the
Continuing surveillance of influenza strains in nature is
spring are incorporated into a vaccine to be given in the fall.
required in order to reformulate the vaccines each year. This
A quote from the U.S. Surgeon General at a meeting of the
surveillance also serves to watch for the possible appear-
Association of State and Territorial Health Officiers in 1957
ance of another killer strain of influenza. An episode that
is worth thinking about: "I am sure that what any of us do,
occurred during the Ford administration, however, illus-
we will be criticized either for doing too much or for doing
trates the potential difficulties of identifying such a strain
too little."
and reacting in time. In February of 1976, a young soldier
at Fort Dix died of influenza and others became seriously
Bird Flu
ill. Tests showed that most of the soldiers were suffering
from the A/Victoria strain of influenza that was epidemic in
A recent scare began when 18 people in Hong Kong
the United States at the time or from adenovirus infection.
became seriously ill from influenza in 1997 and 6 died. The
However, the soldier who died and three other soldiers who
culprit was an avian influenza (H5N1) that was epidemic
were ill were infected with an influenza strain that was epi-
in birds being sold in the markets for food. Avian viruses
demic in pigs, referred to as swine flu. Serology studies indi-
do not normally infect people, and there was fear that an
cated that 200 or more other soldiers had been infected by
avian virus had made the jump to humans and might cause
this virus as well, showing that the virus was being transmit-
an epidemic of lethal influenza. The Hong Kong authorities
ted from person to person. The swine flu virus was closely
destroyed 1.6 million domestic birds in order to eradicate the
related to the 1918 pandemic virus, and is thought to have
epidemic in birds. No human-to-human transmission took
been introduced into pigs in 1918 from humans and to have
place and the virus disappeared. In 2002, however, H5N1
continued to circulate in pigs after it had died out in humans.
virus reappeared and by 2006 it has spread throughout Asia
Could it be possible that the 1918 virus had reappeared as
and into Africa and Europe. This virus has a mortality rate of
an epidemic virus in humans? The decision was made by
about 50% in humans and more than 140 people have died
President Ford, in consulation with leading scientists, to
of H5N1 infection as of this date. There is no person-to-per-
begin a crash program to develop a vaccine against swine flu
son transmission to date, but there is concern that the virus
and to begin to immunize the American population. It was
might mutate and cause a wide and devastating pandemic
thought, with some justification, that to wait for an epidemic
of influenza. This subject is considered at more length in
to begin before an immunization program was undertaken
Chapter 8.
would mean that it would be too late to be effective, given
the speed with which influenza epidemics spread. Further,
Influenza B and C Viruses
influenza is usually epidemic in winter, and the early detec-
Humans are the reservoir of influenza B virus. It causes
tion of this virus made possible the preparation of a vac-
influenza in humans but there exists only one subtype and
cine before the (next) winter flu season set in. Forty million
antigenic shift does not occur. Antigenic drift does occur,
Americans were immunized against swine flu. No epidemic
and the virus can cause epidemics of serious illness that
of swine flu developed, however, and litigation began. The
result in increased mortality, particularly among the elderly,
pharmaceutical companies had been reluctant to participate
as shown in Fig. 4.17. For this reason, the current strain of
in the program, pointing out that at any one time a certain
circulating influenza B is included in the annual flu vaccine.
fraction of Americans would develop encephalitis or rheu-
However, wide-ranging pandemics do not occur and the
matoid arthritis or any one of hundreds of other diseases.
virus is therefore not as much of a problem as influenza A.
If disease developed in proximity to receiving a new and
Less attention has accordingly been given to the study of this
relatively untested vaccine, a lawsuit would certainly fol-
virus. Influenza C is not a serious human pathogen and has
low and the potential damages were enormous. The program
been even less well studied.
could only advance when Congress agreed to indemnify the
Search WWH :