has one membrane-spanning region. These two proteins are
Coronaviruses for many other animals are known, includ-
important for virion morphogenesis.
ing mice, chickens, pigs, and cats. Diseases associated with
Some coronaviruses belonging to group 2 also possess
various coronaviruses in these animals include respiratory
a fourth envelope protein, a hemagglutinin-esterase (HE).
disease, gastroenteritis, hepatitis, and a syndrome similar
Remarkably, this protein appears to be homologous to the
to multiple sclerosis of humans, as well as other illnesses.
H-E of influenza C virus (described in the next chapter).
Mouse hepatitis virus has been particularly well studied as a
It appears that recombination between a coronavirus and an
model for the genus. Feline infectious peritonitis (FIP) coro-
influenza C virus occurred that led to exchange of this protein.
navirus has also been intensively studied. This virus causes
Because only some coronaviruses possess HE, whereas all
a severe infection of cats that is often fatal. It is immunosup-
influenza C viruses possess it, the simplest hypothesis is that
pressive and the high fatality rate results from an inability
HE was an influenza C protein that was acquired by a coro-
to control the infection such that viral replication eventu-
navirus. Presumably, this acquisition was maintained because
ally reaches very high levels. Vaccination of cats with either
it extended the host range of the coronavirus by allowing it to
structural proteins or nonstructural proteins did not protect
infect cells by binding to 5-N-acetyl-9-O-acetyl-N-neuraminic
the animals. In fact, vaccination with structural proteins
acid, a type of sialic acid, or to related sialic acids, depending
made subsequent infection with live virus more severe.
on the specificity of the HE. Maintaining the HE protein has a
Persistent infection was observed in most animals, and there
cost for the virus. Mouse hepatitis virus loses HE when passed
is evidence that virus remains even in animals that eventu-
in culture, demonstrating that it is not needed for replication in
ally control the infection since virus replication can resume
cultured cells and that virus without the gene outcompete virus
if the animals are immunosuppressed. There are some paral-
with the gene. In mice, MHV with HE is more virulent than
lels with SARS infection of humans, in that T-cell lympho-
virus without this gene and can spread more easily to the ner-
penia and viral persistence have been reported.
vous system. Importantly, the HE gene is conserved in MHV
strains isolated in the field, showing that this gene confers a
FAMILY AR TERIVIRIDAE
selective advantage upon the virus.
The family Arteriviridae contains four viruses, which
Diseases Caused by Coronaviruses
are listed in Table 3.14. There are no known human viruses
Until recently, coronaviruses were considered to cause
in the family, but it is of interest because it represents an
only mild disease in humans. Two human coronaviruses were
intermediate between the coronaviruses and other envel-
known, HCoV OC43 (group 2A) and HCoV 229E (group 1).
oped (+)RNA viruses. The genome of equine arterivirus
These viruses are responsible for about 25% of human colds
is illustrated in Fig. 3.39. The arteriviruses have a 13-kb
and are spread by a respiratory route. Unlike rhinoviruses,
genome that is very similar in organization and expres-
they cause not only upper respiratory tract infections but
sion strategy to that of coronaviruses. The virion (60 nm)
sometimes lower respiratory tract infections as well, which
is enveloped, as are the coronaviruses, but the nucleo-
are more serious. There is weak evidence that coronaviruses
capsid, which is poorly defined, is probably icosahedral
might also cause gastroenteritis in humans, because there
rather than helical. The arteriviruses could have arisen by
have been reports of coronaviruses in the stools of people
the acquisition of new structural proteins by a coronavirus
suffering from gastroenteritis. The status of coronaviruses
(or vice versa). The existence of this family, which appears
as human disease agents changed with the recent isolation of
to be a coronavirus with structural proteins that lead to
two new human coronaviruses, NL63 (group 1) and HKU1
icosahedral symmetry rather than helical symmetry, illus-
(group 2A), and with the 2003 epidemic of SARS (group
trates a problem for taxonomy. The ICTV has classified
2B). NL63 is an important cause of severe lower respira-
these viruses as a distinct family, but created the order
tory tract infections in both adults and children. HKU1 has
Nidovirales to indicate their relation to the coronaviruses.
been isolated from adults with pneumonia. SARS causes an
The four arteriviruses are lactate dehydrogenase-elevat-
atypical pneumonia that carries a 10% fatality rate. It is a
ing virus of mice (LDV), equine arteritis virus (EAV),
bat virus that jumped to humans in China, causing an epi-
simian hemorrhagic fever virus (SHFV), and porcine repro-
demic of SARS that began in 2002. In 2003 it was spread
ductive and respiratory syndrome virus (PRRSV). The pri-
around the world by air travelers, eventually causing more
mary target cells in their respective hosts are macrophages,
than 8000 cases of human disease and almost 800 deaths. It
and all are associated with persistent, long-term infections.
was eventually controlled by culling of animals that served
LDV causes a lifelong infection of mice that requires special
as intermediates in passing the virus from bats to humans,
care to detect. EAV causes epizootics of subclinical or mild
and by quarantine procedures. There is concern that epidem-
respiratory diseases in adult horses. Infection can lead to
ics will recur since the virus is widely distributed in China.
abortions in pregnant mares, and infection of young horses
This topic is covered in more detail in Chapter 8.
causes a more serious illness. The virus persists for long
TABLE 3.14 Arteriviridae and Roniviridae
Virus name
Usual
World
Genus/members
abbreviation
host(s)
Transmission
Disease
distribution
Arteriviridae
Arterivirus
Equine arteritis
EAV
Horses
Aerosols, contact
Fever, necrosis of
Worldwide
arteries, abortion
Porcine reproductive and
PRRSV
Pigs
Oralfecal?
Infertility, respiratory
?
respiratory syndrome
distress
Lactic dehydrogenase-elevating
LDV
Mice
Biting
?
?
Simian hemorrhagic fever
SHFV
Monkeys
Biting
Hemorrhage
?
Roniviridae
Okavirus
Gill-associated virus
GAV
Invertebrates
Vertical, horizontal
Chronic subclinical,
Asia and
(prawns)
also acute necrosis of
Australia
lymphoid organ
CAP
An
G enome RNA
ORF1a
ORF1b
An
Gp2
An
Gp3
An
Gp4
mRNAs
An
Gp5
An
M
An
N
0
5
9.5
10
10.5
11
11.5
12
12.5 kilobases
p29
p61
p22
p31 p7
p41
p80
p50
p26
p12
nsP1
nsP2
nsP3 nsP4
nsP5
Protease Cleavages
Enzyme Motifs
Coding Domains
Nonstructural proteins
nsP2 autoprotease
Polymerase (GDD)
Virion nonglycosylated proteins
nsP1 autoprotease
Helicase
Virion glycoproteins
nsP4pro
Zinc finger
3C-like cysteine protease
nsP4pro(predicted)
(nsP2)
unknown (cellular?)
Papain protease (nsP1)
Serine protease
(nsP4)
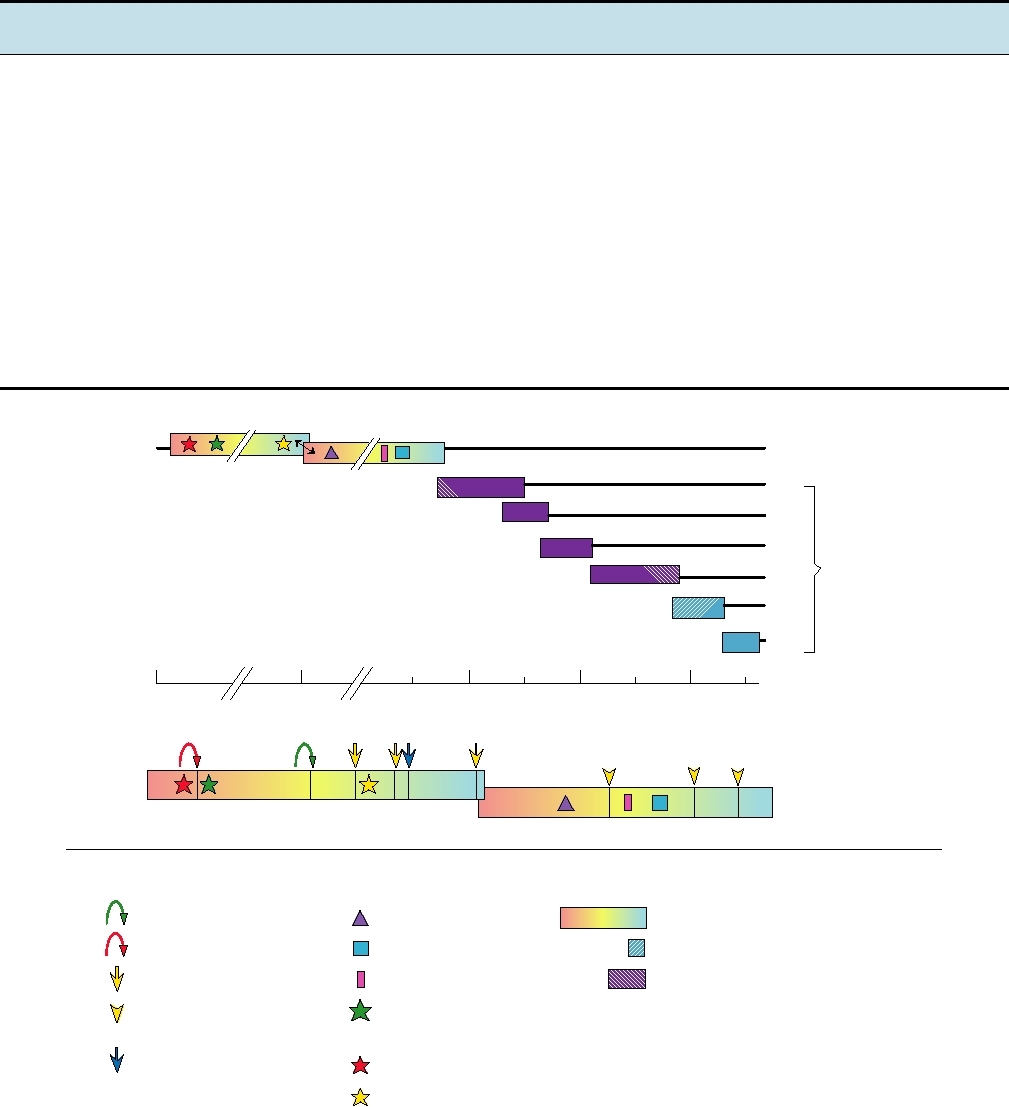
FIGURE 3.39 Upper panel: genome organization of an arterivirus, equine arteritis virus. ORF1a and ORF1b encode
components of the viral replicase and are translated as a polyprotein with ribosomal frameshifting at the arrow. The remaining
viral components are encoded in a nested set of mRNAs. The hatched proteins are polypeptides found in virions. Lower panel:
proteolytic processing of the equine arteritis virus ORF1ab polyprotein. Positions of motifs of proteases, polymerase, zinc finger,
and helicase are indicated with various symbols. Arrows are color coded to indicate cleavage by the corresponding protease.
Arrowheads are predicted cleavages. Blue arrowhead is a cleavage site possibly cleaved by a cellular protease. Adapted from de
Vries et al. (1997) and den Boon et al. (1991).
Search WWH :