ORF 1
ORF 2
An
?
genome
ORF 1a
ORF 1b
An
mRNA
Serine Proteinase
Nucleocapsid protein
Polymerase (GDD)
Nonstructural proteins
Frameshift
FIGURE 3.15 Genome organization of a human astrovirus. ORF 1a is in a different reading frame than ORF 1b.
Ribosomal frameshifting (arrows) during translation is required to produce the ORF 1b protein. Adapted from Fauquet
et al. (2005) p. 860.
TABLE 3.9 Viruses Causing Acute Diarrhea
Virusa
Nucleic acid
Family
Host range
Single-stranded,
Caliciviridae
Sapporo, Norwalk feline
Humans, cattle, swine, chickens, dogs, cats
plus-sense RNA
calicivirus
Astroviridae
Numerous astroviruses
Humans, cattle, swine, cats, dogs, avian species
Swine, cattle, foals, mice, rabbits, dogs, cats turkeys, (humans?)b
Coronaviridae
PEDV, TGEV and others
Numerous toroviruses
Cattle, horses (goats, sheep, swine, rabbits, mice, humans?)
Flaviviridae
Pestivirus BVDV
Cattle
Picornaviridae
Aichi, human parechovirus 1
Humans
Single-stranded,
Paramyxoviridae
Canine distemper
Dogs
minus-sense RNA
Newcastle disease
Chickens, fowl
Double-stranded RNA
Reoviridae
Rotavirus A
Mammalian and avian species, humans
Rotavirus B
Swine, cattle, sheep, rodents, humans
Rotavirus C
Swine, ferrets, humans
Rotaviruses D, F, G
Avian species
Rotavirus E
Swine
Single-stranded DNA
Parvoviridae
Numerous parvoviruses
Cattle, cats, dogs, mink, (humans?)
Double-stranded DNA
Adenoviridae
Human Ad40,41
Humans
a
Abbreviations: PEDV, porcine epidemic diarrhea virus; TGEV, transmissible gastroentieritis virus; BVDV, bovine viral diarrhea virus.
b
The role of the listed viruses in causing diarrhea has not been proven for species listed in parentheses.
Source: Adapted from Granoff and Webster (1999), p. 442.
FAMILY TOGAVIRIDAE
be other small viruses that cause gastroenteritis in humans.
Virus particles have been seen in stools of humans suffer-
ing from gastroenteritis that have not as yet been charac-
The family Togaviridae contains two genera, genus
terized and are referred to simply as SRVs (small, round
Alphavirus and genus Rubivirus. The family name comes
viruses). Some of these may be members of families not
from the Latin word for cloak, and the name was given to
yet characterized.
them because they are enveloped. The 30 alphaviruses have
Alphavirus (Sindbis virus)
An genome RNA
CAP
nsP1
nsP2
nsP3
nsP4
An 26S mRNA
CAP
C
E2
E1
E3
6K
Rubivirus (rubella virus)
An
CAP
genome RNA
p150
p90
24S mRNA
CAP
An
C
E2
E1
Motifs
Proteolytic
Coding domains
Cleavages
Helicase
Nonstructural proteins
Capsid autoprotease
Nucleocapsid protein
Polymerase (GDD)
nsP2 or p150 protease
Opal codon
Glycoproteins
Papain protease
Signalase
Serine protease
Furin
Methyl transferase
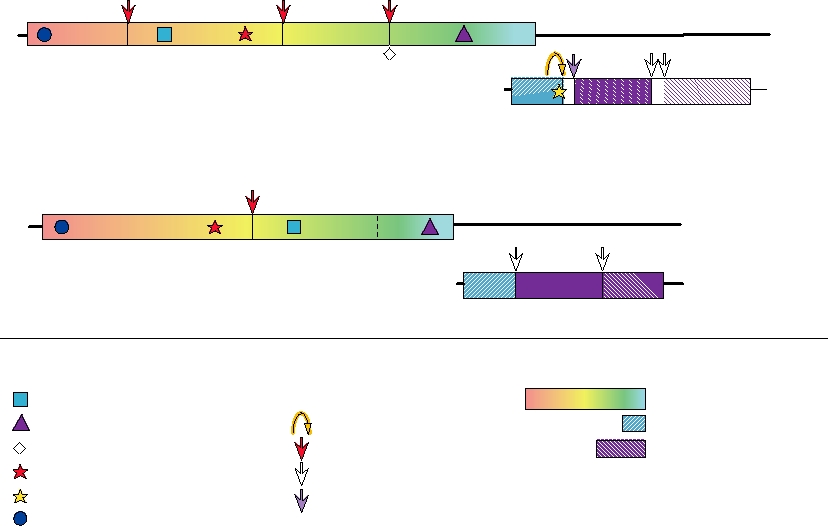
FIGURE 3.16 Genome organizations of the Togaviridae. A number of protein motifs are indicated, as well as the enzymes
responsible for the proteolytic cleavages. The opal codon shown between nsP3 and nsP4 is leaky and is not present in all
alphavirus isolates; readthrough produces small amounts of nsP4. Adapted from Strauss and Strauss (1994) Figure 34.
a (+)RNA genome of about 12 kb, whereas rubella virus, the
selection of these viruses is presented in Table 3.10. Because
only member of the Rubivirus genus, has a genome of 10 kb.
of their importance as disease agents and aided by the fact that
The genomes of alphaviruses and of rubella virus are organ-
alphaviruses grow well in cultured cells and in animal mod-
ized in a similar fashion, as illustrated in Fig. 3.16. The viri-
els, this group of viruses has been well studied. The genomes
ons of the two groups are also roughly similar in size (70 nm
of many of them have been sequenced in their entirety. All
for alphaviruses, 50 nm for rubiviruses) and structure (icosa-
members of the genus are closely related and share extensive
hedral nucleocapsids surrounded by a lipoprotein envelope).
amino acid sequence identity, with the most distantly related
Structures of alphaviruses are illustrated in Figs. 2.5, 2.14,
alphaviruses sharing about 40% amino acid sequence identity
and 2.18. However, although the two genera exhibit simi-
on average and viruses belonging to the same lineage sharing
larities, they are only distantly related. As an historical foot-
higher sequence identity. A dendrogram that illustrates their
note, the flaviviruses, described after the togaviruses, were
relationships is shown in Fig. 3.17. A number of lineages or
once classified as a genus within the Togaviridae. When
clades are present, including a clade of aquatic viruses, a clade
sequences of alphaviruses and flaviviruses were determined,
of encephalitic viruses (EEE, VEE), the Sindbis clade (which
however, they were found to be unrelated and the flavivi-
includes Aura virus and many strains of Sindbis virus), the
ruses were placed into a new family.
SFV clade (which includes many viruses which cause arthri-
tis including polyarthritis), and a clade of recombinant viruses
(the WEE lineage). The dendrogram illustrates the interesting
Genus Alphavirus
fact that during evolution of alphaviruses, there was a singu-
The alphaviruses have a worldwide distribution. They get
lar recombination event between Eastern equine encephali-
their name from the Greek letter alpha because they were
tis virus and a Sindbis-like virus to produce Western equine
once known as the Group A arboviruses. Many cause impor-
encephalitis virus, which subsequently evolved into a number
tant illnesses in humans, and information for a representative
of different viruses (the WEE lineage). Recombination events
TABLE 3.10 Togaviridae
Virus name
World
Genus/members
abbreviation
Usual host(s)
Transmission
Disease
distribution
Alphavirus
Mammalsa, Birds
Sindbis
SINV
Mosquito-borne
Arthralgia, rash, fever
Old World
Mammalsa
Semliki Forest
SFV
Mosquito-borne
Arthralgia, fever
Africa
Mammalsa
Ross River,
RRV, BFV
Mosquito-borne
Polyarthritis, fever, rash
Australasia
Barmah Forest
Ft. Morgan
FMV
Birds
Vectored by
?
North America
swallow bug
Chikungunya,
CHIKV,
Humans
Mosquito-borne
Arthralgia, fever
Africa
O'Nyong-nyong
ONNV
Mammalsa
Mayaro
MAYV
Mosquito-borne
Arthralgia, fever
South America
Mammalsa, Birds
Eastern, Western,
EEEV, WEEV,
Mosquito-borne
Encephalitis
Americas
Venezuelan equine
VEEV
encephalitis
Salmon pancreas
SPDV
Fish
No arthropod vector
disease virus
Rubivirus
Rubella
RUBV
Humans
No arthropod vector
Rash, congenital
Americas, Europe
abnormalities
a
Humans can be infected by these viruses, but humans are not the primary mammalian reservoir.
A
B
RUBV
RUBV
Aquatic Virus
SPDV
SPDV
Aquatic Virus
Clade
Clade
SDV
SDV
OCKV
AURAV
Sindbis
Sindbis
SINV
OCKV
Clade
Clade
WEEV
SINV
AURAV
Equine
Equine
VEEV
VEEV
Encephalitis
Encephalitis
EEEV
EEEV
Clade
Clade
WEEV
BFV
BFV
MIDV
MAYV
MAYV
SFV
SFV
Semliki
Semliki
RRV
Forest
Forest
RRV
SAGV
Clade
Clade
SAGV
CHIKV
CHIKV
ONNV
ONNV
SESV
0.1
0.1
Genetic distance
Genetic distance
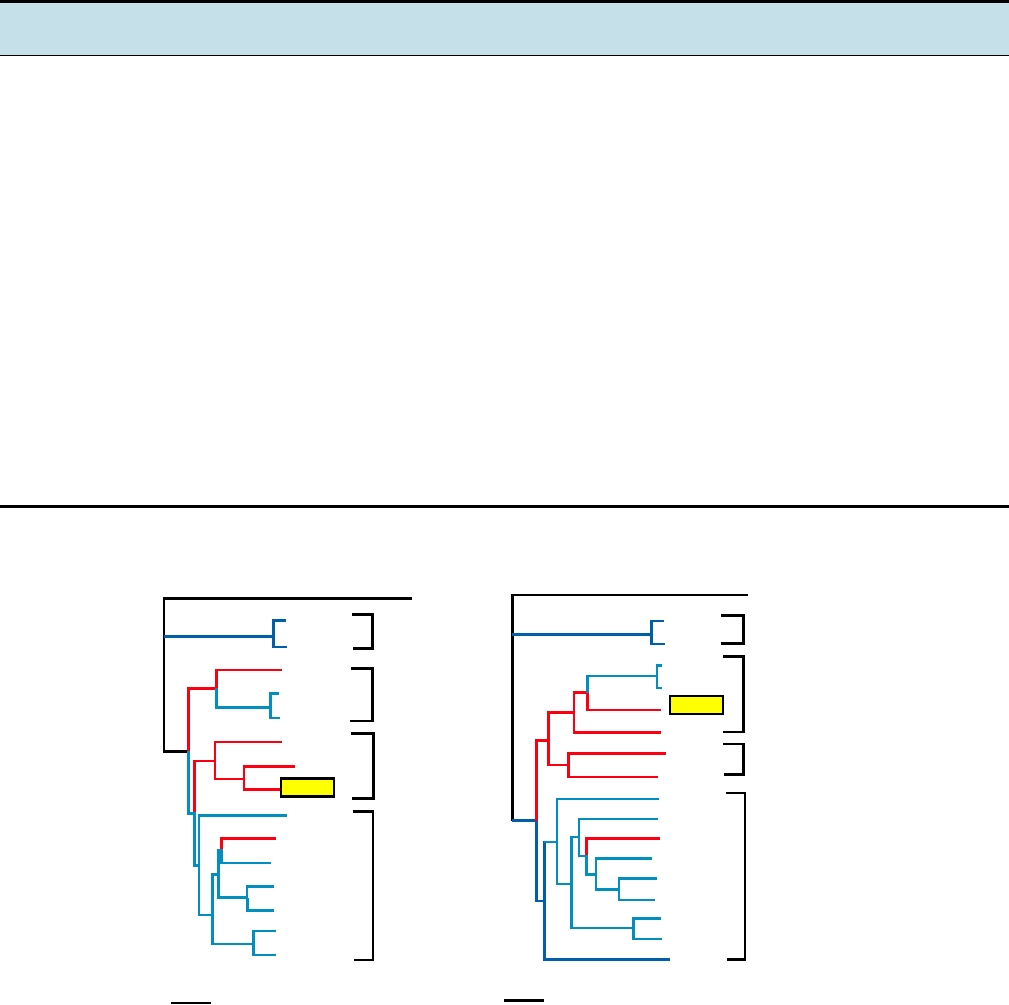
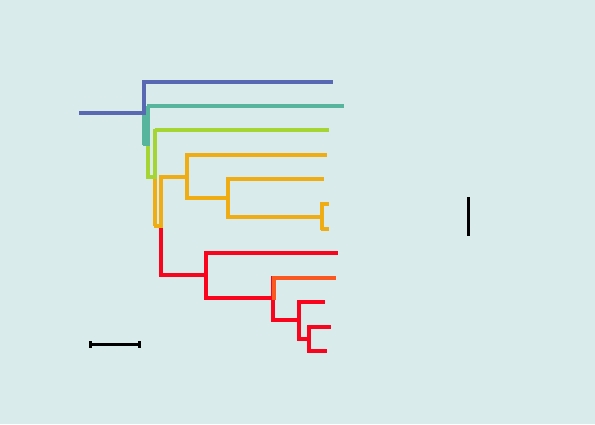
FIGURE 3.17 Phylogenetic trees of the alphaviruses, constructed using the neighbor-joining method from the distances
computed using Clustal X v 1.81 software, with the sequences from rubella virus as outgroup. The Old World viruses are
shown in blue, the New World viruses in red. The vertical distances are arbitrary, but the lengths of the horizontal branches
indicate the number of amino acid substitutions along the branch. (A) A tree derived from the amino acid sequences of
nonstructural proteins nsP1 through nsP4, comprising roughly 2475 amino acids (B) A tree derived from the sequences of
the virion structural proteins or roughly 1245 amino acids. The trees are very similar in architecture with the exception of
the position of WEE (boxed), whose nonstructural proteins are most closely related to EEE, but whose structural proteins
resemble SIN, indicating that a recombinational event has taken place to produce WEE. Note that Fort Morgan, Buggy Creek,
and Highlands J (not shown) are three recombinant New World viruses closely related to WEE. Most virus abbreviations are
found in Table 3.10; AURAV, Aura; MIDV, Middelburg; SDV, sleeping disease; OCKV, Ockelbo; SAGV, Sagiyama; SESV,
Southern elephant seal virus. These trees were adapted from Luers et al. (2005) with permission.
in which the recombinant virus persists and prospers as a
Replication of the viral RNA takes place in association
distinct virus appear to be uncommon to rare, but there is
with cellular membranes. Small spherical invaginations
much evidence that recombination has played an important
form in the membranes, induced by viral proteins, in which
role in the evolution of viruses. The dendrogram also illus-
replication occurs. Protein nsP1 has been shown to interact
trates the fact that there have only been a limited number of
with membranes by means of a specific domain within the
transfers of alphaviruses between the Americas and the Old
protein, and it is assumed that this association is important
World. In fact, three transfers between the Americas and the
for the membrane association of the replication machinery.
Old World are sufficient to explain the dendrogram, and the
Studies of the viral nonstructural protease have shown that
majority of members of the three major lineages are restricted
the cleavages that process the polyprotein control viral RNA
to either the Americas or to the Old World. This contrasts
replication. During or shortly after translation, the full-length
with many virus families, where individual viruses are often
polyprotein precursor (called P1234) cleaves itself in cis
worldwide in distribution and evidence is abundant for the
to produce P123 and nsP4. These form an RNA synthetase,
mixing of lineages between the two hemispheres.
probably together with cellular proteins, that can make com-
plementary (-)RNA from the genomic RNA template, but
which cannot make (+)RNA efficiently. Subsequent cleav-
Expression of the Genome
age of P123 in trans, between nsP1 and nsP2, gives rise
Alphaviruses enter a cell via endosomal uptake and fuse
to a synthetase that can make both (+)RNA and (-)RNA.
with the endosomal membrane upon exposure to a suita-
A second cleavage between nsP2 and nsP3 gives rise to a
bly low pH that differs somewhat from virus to virus. The
synthetase that can make only (+)RNA. Thus, (-)RNA tem-
capsid protein has an affinity for ribosomes and there is
plates are made early, but as infection proceeds and the con-
evidence that ribosomes help disassemble the nucleocap-
centration of protease builds up, trans cleavage occurs and
sid upon its entry into the cytoplasm by binding the capsid
(-)RNA synthesis is shut down (Fig. 3.18). After this time,
protein. Release of the genomic RNA, which is capped and
plus-strand RNA synthesis continues from the preformed
polyadenylated, is followed by its translation into a non-
minus-strand templates but no further increase in the rate
structural polyprotein that is cleaved into four polypep-
of RNA synthesis takes place. This control mechanism may
tides by a viral protease (Fig. 3.16). Activities present in
have evolved not only to make the infection process more
these proteins include a capping activity in the N-terminal
efficient, since genomic RNA for progeny virions and sub-
protein (nsP1); helicase, papain-like protease, and RNA
genomic RNAs for translation of structural proteins required
triphosphatase activities in nsP2; and RNA polymerase in
nsP4. A viral encoded capping activity is required to cap the
viral mRNAs (the genomic RNA and a subgenomic RNA)
because replication occurs in the cytoplasm and the virus
does not have access to cellular capping enzymes. The RNA
103
triphosphatase in nsP2 is required to remove the terminal
10
100
phosphate in the 5′ triphosphate on the RNA in order to
prepare the RNA for capping by the viral enzyme, the RNA
102
helicase is needed to unwind the RNAs during replication,
and the protease is required to process the precursor poly-
protein. The RNA polymerase is needed to synthesize viral
10
1
10
RNA. All four nonstructural proteins are required to synthe-
size the viral RNA. Replication of the RNA and synthesis of
a subgenomic RNA follow the pattern illustrated schemati-
1
cally in Figure 1.9B.
The function of the phosphoprotein nsP3 in RNA replica-
tion is unknown. It is phosphorylated on several threonines
4
8
12
16
and serines in a nonconserved domain in the C-terminal
Hours post infection
region of the protein. The N-terminal domain of nsP3 is a
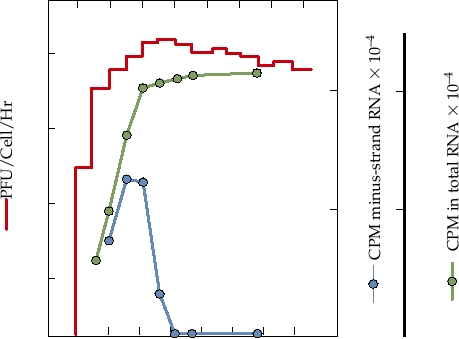
FIGURE 3.18 Growth curve of Sindbis virus infection in chicken cells at
conserved domain (often referred to as the X domain) that is
30°C. At the times shown, the medium was harvested and replaced with fresh
medium, and the titer was determined. The red line shows release of infectious
also present in a number of other viruses (rubella virus, hep-
virus into the culture fluid. For determining the rate of RNA synthesis, cells
atitis E virus, coronaviruses) as well as being widely distrib-
infected as for virus assay were pulsed with radioactive uridine for 1 hour
uted in bacteria, archae, and eukaryotes. The virus domain
at the times shown. Monolayers were harvested and incorporation into
shares up to 3540% amino acid sequence identity with the
total RNA (green line) and minus-strand RNA (blue line) were determined.
cellular homologues, whose function is unknown.
Adapted from Strauss and Strauss (1994) Figure 5.
for assembly of progeny virions are needed in much larger
P123 and its cleavage products may serve to accelerate
quantities than minus-strand templates, but also to control
the rate of RNA synthesis. There is evidence from genetic
the virulence of the virus. In the case of alphaviruses, it is
studies that domains in nsP1 and nsP2, among others, are
particularly important to control the virulence of the virus
required for the recognition of viral promoters and the initia-
in the mosquito vector. As discussed in more detail later,
tion of RNA synthesis, and additional helicase activity could
it is important that the infection process in the mosquito be
also speed up the rate of RNA synthesis.
tightly controlled, and shutting down minus-strand RNA
During infection by alphaviruses, a subgenomic mRNA
synthesis not only prevents further exponential increase in
is produced that serves as the message for the production of
virus replication but also allows downregulation of virus
the structural proteins of the virus, which consist of a cap-
replication as minus-strand templates decay. It also has the
sid protein and two glycoproteins. The 4.1-kb subgenomic
effect that the infected cell becomes resistant to superinfec-
RNA is synthesized by the viral replicase from the (-)RNA
tion by the same or a related virus because no (-)RNA tem-
template using an internal promoter of 24 nucleotides. The
plates can be made, which could be especially important in
activity of this core 24-nucleotide promoter is increased by
the mosquito vector. The resistance to superinfection by the
enhancer sequences present in the 100 nucleotides upstream
same or related viruses is called superinfection exclusion or
of the core promoter. The structural proteins are translated
homologous interference.
as a polyprotein and cleaved by a combination of viral
The rate of cleavage of the early synthetase that makes
and cellular enzymes. The capsid protein is itself a serine
(-)RNA to convert it into one that can make (+)RNA is
autoprotease that cleaves itself from the N terminus of the
thought to be controlled in part by a leaky stop codon
nascent polyprotein. It has a fold that is similar to that of
between nsP3 and nsP4 that is present in most, but not all,
chymotrypsin (Fig. 2.14B), suggesting that it was derived
alphaviruses (Fig. 3.16). Termination at this codon produces
from a cellular serine protease during evolution of the
P123, which can act in trans as a protease but cannot act as
virus. After release of the capsid protein, N-terminal signal
a synthetase because it lacks the nsP4 RNA polymerase. In
sequences and internal signal sequences in the glycoprotein
addition to a more rapid buildup of protease that accelerates
polyprotein precursor lead to its insertion into the endo-
the rate of conversion to (+)RNA synthesis, this additional
plasmic reticulum (Figure 3.19). This precursor is cleaved
E2
E1
PE2
E1
CHO
NH3+
NH3+
NH3+
NH3+
COO-
Lumen
Acylation
Phosphorylation?
ER membrane
Heterodimerization
+++
PE2 cleavage
COO-
COO-
Cytoplasm
+++
6K
Sites of fatty acid acylation
Signalase
Stop transfer signal
Fatty acid chain
Golgi protease (Furin?)
Signal sequence
CHO
Carbohydrate chain
Phosphorylation
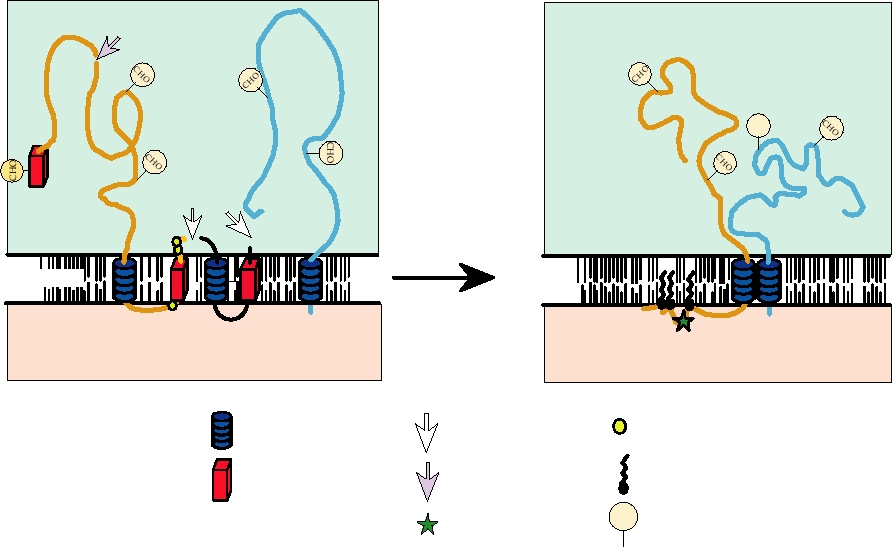
FIGURE 3.19 Schematic diagram of the configurations of glycoproteins PE2 and E1 in membranes. The left panel shows
the configuration of PE2 immediately after signalase cleavage from 6K, with the C terminus in the lumen of the ER. The
right panel shows the configuration of the E2-E1 heterodimer after phosphorylationdephosphorylation with the C terminus
in the cytoplasm. Adapted from Strauss and Strauss (1994), Figures 7 and 9.
by signalase, a cellular enzyme that resides in the lumen of
the genomic RNA and for genomic RNA synthesis from the
the endoplasmic reticulum, to produce glycoprotein PE2
antigenomic template. The genomic RNA (and presumably
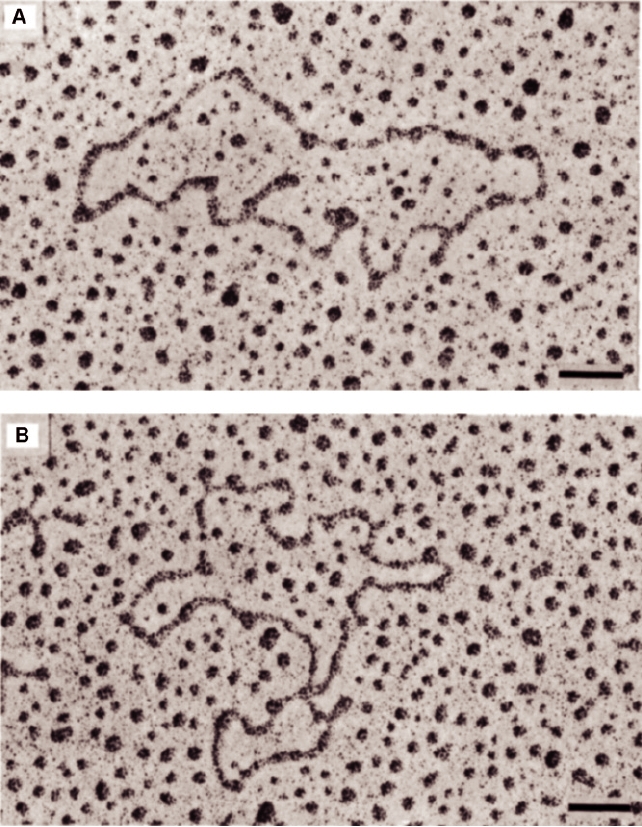
(a precursor to glycoprotein E2), 6K (a small hydrophobic
the antigenomic RNA as well) is known to cyclize in the
peptide located between E2 and E1), and glycoprotein E1.
absence of protein (Fig. 3.21), thus bringing the sequence
PE2 and E1 quickly form a heterodimer. At some time, the C
elements at the two ends of the molecule into close proxim-
terminus of PE2/E2 is withdrawn from the lipid bilayer into
ity, allowing the viral replicase to interact with both ends of
the cytoplasm, where it can interact with the capsid during
the RNA at once when initiating synthesis of new RNA. It
virus assembly. During transport of the heterodimer to the
seems likely that this mechanism evolved so that only full-
cell plasma membrane, PE2 is cleaved by another cellular
length RNA molecules can be replicated. This eliminates
enzyme, furin or a furin-like enzyme, to form E2 and E3. E3
replication not only of the subgenomic RNA but also of any
is a small glycoprotein that in most alphaviruses is lost into
broken RNA molecules.
the culture fluid.
It is noteworthy that the RNAs of many, perhaps most,
RNA viruses, both plus stranded and minus stranded, cyclize
at some stage of RNA replication or translation. In some
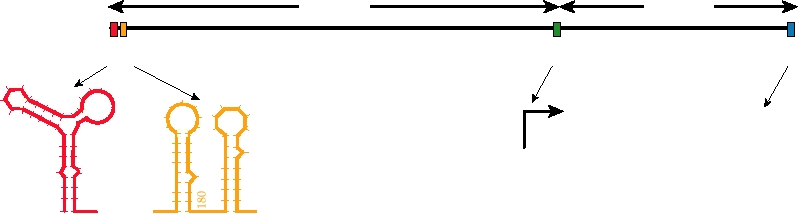
Viral Promoters
cases, as in the alphaviruses, cyclization occurs by means
The replication of alphaviral RNA requires the recognition
of complementary sequences near the ends of the RNA and
of specific promoters in the viral RNA by the viral RNA
requires no protein to stabilize the interaction. In other cases,
synthetase. These promoters act in cis, that is, they must
the interactions of the complementary sequences near the
be present in the RNA to be used as a template, and both
ends of the RNA are stabilized by the binding of viral or cel-
viral and cellular proteins may be involved in the recognition
lular proteins. And in still other cases, cyclization is effected
of these promoters. Four alphavirus promoters, or compo-
by cellular proteins. In one scenario, a cellular protein such
as the poly(A)-binding protein binds to the 3′ poly(A) tract
nents of promoters, are illustrated in Figure 3.20. The best
understood of these promoters is that for the production of the
and another cellular protein, such as one that binds to transla-
tion initiation signals, binds near the 5′ end of the molecule.
subgenomic mRNA for the structural proteins. The basal pro-
moter consists of 24 nucleotides, of which 19 are upstream
These two cellular proteins then interact with each other to
of the transcription start site and 5 are copied into the sub-
hold the two ends of the RNA near one another.
genomic RNA. This subgenomic promoter can be placed in
front of any RNA sequence and the viral synthetase will use
Assembly of Progeny Virions
it to synthesize a subgenomic mRNA. This property of the
promoter has made alphaviruses useful as expression vectors
The structure of alphaviruses has been described in
(Chapter 11).
Chapter 2. PE2-E1 heterodimers form in the endoplasmic
The promoters for synthesis of full-length genomic RNA
reticulum and are transported to the cell surface. During
and the antigenomic (-)RNA template are less well under-
transport, they are cleaved to form E2-E1 heterodimers. The
stood. A linear sequence element at the 3′ end of the (+)RNA
PE2-E1 heterodimer is more stable to acidic pH than is the
and two elements at or near the 5′ end of the genomic RNA
E2-E1 heterodimer and survives the mildly acidic pH of
that can form stem-loop structures (Fig. 3.20) are required
transport vesicles. Cleavage is necessary to prime the E2-E1
for the efficient synthesis of both the antigenomic RNA from
heterodimer for disassembly upon entry into a susceptible
3
5
7.6 kb
4.1 kb
An
CAP
12
3
4
20
30
26S RNA
10
AUUUUGUUUUUAAUAUUUC
170
190
160
[4]
200
ACCUCUACGGCGGUCCUAA AUAGG
40
19 NT CONSERVED
[3]
SEQUENCE
CAP
CONSENSUS JUNCTION
[1]
[2]
SEQUENCE
CONSERVED STRUCTURES
FIGURE 3.20 Promoters in the alphavirus genome. Adapted from Strauss and Strauss (1994), Figure 18.
FIGURE 3.21 Electron micrographs of Sindbis virus 49S genomic RNA. The RNA was treated with 0.5 M glyoxal in
0.1 M phosphate buffer for either 30 min (A) or 40 min (B) at 35°C before spreading. Scale bar is 100 nm. Adapted from
Frey et al. (1979) Figure 3.
cell, which is required for formation of E1 homotrimers that
interactions. The diameter of the assembled virion is 70 nm
are responsible for fusion. During transport or virus assem-
(Figs. 2.5 and 2.14A).
bly, three E2-E1 heterodimers trimerize to form the spikes
The 6K protein is required for efficient assembly of
found in the virion. Virions normally mature when a preas-
virions. Virions that appear to be normal in every way will
sembled nucleocapsid consisting of the genomic RNA and
assemble in the complete absence of 6K but only inefficiently
240 copies of capsid protein buds through the cell plasma
and virus that lacks the 6K gene produces only low yields.
membrane to acquire a lipoprotein envelope containing 240
It has been shown that 6K expressed alone will form ion
copies of the E1-E2 heterodimer (Fig. 2.25C). The free energy
channels, but whether this is important for virus assembly
for budding is provided by lateral interactions between the
is not known.
viral glycoproteins as they assemble around the nucleocap-
sid and by the interaction of the C-terminal domain of glyco-
Alphaviruses Are Arboviruses
protein E2 with a docking site on each nucleocapsid protein
molecule. The assembled virion has icosahedral symmetry
All alphaviruses are arboviruses (arthropod-borne
in which the symmetry axes of the nucleocapsid are coordi-
animal viruses), with the probable exception of the
nated with those of the glycoprotein shell by the E2capsid
newly described fish viruses, and were once referred to as
4
3
1
2
5
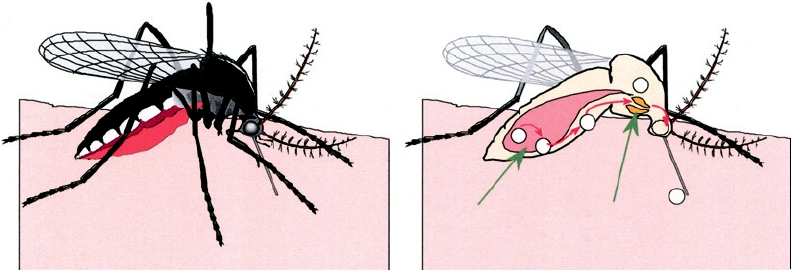
Mesenteron
Salivary Glands
A. A female mosquito takes a blood meal
B. Cutaway view of the mosquito showing
steps in the replication and transmission
of an arbovirus
FIGURE 3.22 Sequential steps necessary for a mosquito to transmit an arbovirus. (1) A female mosquito ingests an
infectious blood meal and virus enters the mesenteron. (2) Virus infects and multiplies in mesenteronal epithelial cells. (3)
Virus is released across the basal membrane of the epithelial cells and replicates in other tissues. (4) Virus infects salivary
glands. (5) Virus is released from the epithelial cells of the salivary glands and is transmitted in the saliva during feeding.
Adapted from Monath (1988).
the Group A arboviruses. In nature, they alternate between
transmission cycle is illustrated, such as that of urban yellow
replication in arthropod vectors, usually mosquitoes, and
fever infection of humans (see the section on flaviviruses
higher vertebrates. A mosquito may become infected on
later). In this cycle, humans are the only vertebrate hosts and
taking a blood meal from a viremic vertebrate, which can
the virus alternates between infection of a human and infec-
have 108 or more infectious particles per milliliter of blood.
tion of the mosquito vector Aedes aegypti. Figure 3.23B
The infection in the mosquito, which is almost asymptom-
illustrates a complex transmission pattern, using as an exam-
atic and lifelong, begins in the midgut and spreads to the
ple the transmission of Eastern equine encephalitis virus in
salivary glands, as illustrated in Fig. 3.22. After infection
North America. This virus has a vertebrate reservoir con-
of the salivary glands, the mosquito can transmit the virus
sisting primarily of migratory songbirds and is transmitted
to a new vertebrate host when it next takes a blood meal,
by the mosquito, Culiseta melanura, a common inhabitant
injecting saliva in the process. Infection in the vertebrate
of freshwater swamps in eastern North America. However,
begins in the tissues surrounding the bite or in regional
the virus is capable of infecting other mosquitoes and has
lymph nodes, but then spreads to other organs. The infec-
even been isolated from naturally infected chicken mites. It
tion is usually self-limited and the vertebrate is capable of
also infects mammals, including humans. On occasion, the
infecting mosquitoes for only a brief time after viremia is
virus breaks out of its enzootic cycle to cause epidemics of
established but before an immune response limits circu-
disease in pheasants, transmitted and maintained by an epi-
lating virus. The necessity to alternate between two such
zootic vector mosquito. Either enzootic or epizootic vectors
different hosts has constrained the evolution of arbovi-
are capable of infecting humans or domestic animals if they
ruses--changes that adapt the virus to one host or that are
invade the areas in which these mosquitoes are present, but
neutral in one host are often deleterious in the alternate
these hosts are usually dead-end hosts and do not further
host. Thus, the evolutionary pressures on arboviruses are
spread the virus.
different from those on viruses such as poliovirus, which
Most alphaviruses are capable of infecting both mam-
infects only primates.
mals and birds, and the nature of the vertebrate reservoir
Different alphaviruses infect different spectra of mosquitoes
depends on the virus or even the strain of virus, which may
and vertebrates in nature. It is useful to distinguish between
differ by geographic location. Thus, for example, Sindbis
reservoir hosts in which the virus is maintained in nature and
virus in nature is normally maintained in birds, which are
dead-end hosts in which infection normally does not lead to
its usual vertebrate reservoir. However, the virus is capable
continuity of the infection cycle. We can also distinguish
of infecting mammals, including humans, and has also been
between enzootic cycles, in which the virus is continuously
isolated from amphibians and reptiles. Numerous species
maintained in nature and which may or may not result in
of mosquitoes form its insect reservoir, but it has also been
disease in the enzootic host, and epizootic cycles, in which
isolated from other hematophagous arthropods, including
the virus breaks out and causes epidemics of disease that
mites. In contrast, Ross River virus is maintained in small
may die out with time. Two types of natural transmission
marsupial mammals in Australia and does not appear to use
cycles are illustrated in Fig. 3.23. In Fig. 3.23A, a simple
birds as hosts.
A. Simple Transmission Cycle
Domestic Vector
Domestic Vector
Primary Vertebrate Host
B.
Complex Transmission Cycle
Epizootic Host
Enzootic Host
Pheasant
Passerine Bird
Enzootic Vector
Epizootic Vector
Enzootic Vector
Epizootic Vector
Dead-End Hosts
Man and Horses
Enzootic Host
Epizootic Host
Passerine Bird
Pheasant
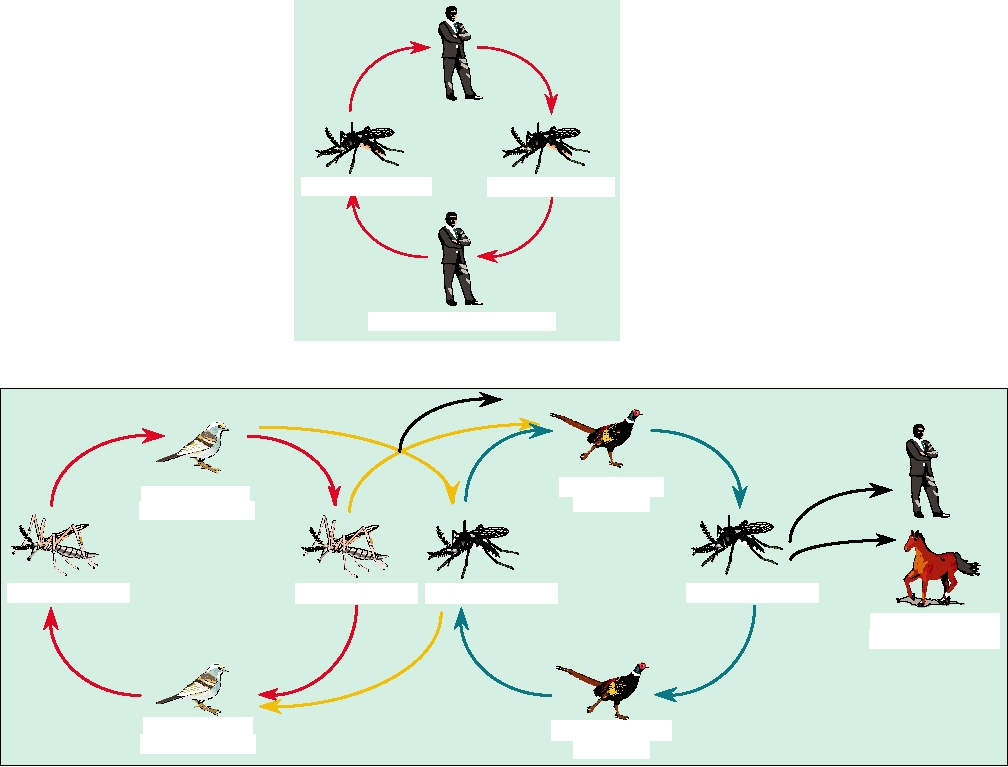
FIGURE 3.23 Two generalized transmission cycles of arboviruses. (A) Simple cycle, such as urban yellow fever,
involving a single vector (Aedes aegypti mosquitoes) and a single vertebrate host (man). (B) Complex cycle, such as that
for Eastern equine encephalitis, where the virus is maintained in an enzootic host (passerine birds) with an enzootic vector
(Culiseta melanura), but can enter an epizootic vector (another insect) and be transmitted to epizootic hosts, and tangentially
to dead-end hosts like man. An intermediate type of cycle is illustrated in Figure 5.12 for Colorado tick fever. Adapted from
Monath (1988) p. 129.
Arboviruses that are transmitted by mosquitoes,
that the female mosquito survive long enough and be healthy
ticks, sand flies, or other bloodsucking arthropods are
enough to take another blood meal, which is required for egg
known from several families of viruses. A selection of
development. The time between infection of the female and
arboviruses from three virus families, most of which cause
the time at which the mosquito is capable of transmitting the
human disease, is listed in Table 3.11, together with the
virus is called the extrinsic incubation period. This period
diseases they cause.
varies widely with temperature, humidity, the virus, and the
mosquito. It can be as short as 2 days or as long as 2 weeks,
Pathology in the Mosquito Vector
although in alphaviruses it is seldom longer than 1 week.
The mosquito vector suffers little pathology upon infec-
Although infection of the mosquito is relatively benign,
tion by an arbovirus. This must be so if the virus is to persist,
recent studies have shown that there is some pathology asso-
because transmission of the virus to a new vertebrate requires
ciated with arboviral infection. There is limited pathology
TABLE 3.11 Representative arboviruses that cause human disease
Predominant disease manifestationsa
Nonfatal
Encephalitis
Hemorrhagic Fever (HF)
systemic
Frequencyb
Frequencyb
%Mortalityd
%Mortalityc
Family/virus
febrile illness
Togaviridae
Chikungunya
Most cases, Ep
Rare, Ep
rare
Mayaro
Most cases, Ep
O'nyong-nyong
Most cases, Ep
Ross River
Most cases, Ep
Sindbis
Most cases, Ep
EEE
Most cases
Rare
5070
0.120 e
VEE
Most cases, Ep
Rare
WEE
Most cases
Rare
510
Flaviviridae
Dengue (14)
Most cases, Ep
Rare, Ep
312
∼9%f
West Nile
Ep
En
3040 g
Japanese encephalitis
<1%
Kyasanur Forest
En
5
En
5
Murray Valley
Ep
2070
Rocio
Ep
13
St. Louis encephalitis
Ep
420
Tick-borne encephalitis
Eastern
Rare
30
Central European
Rare
110
Omsk hemorrhagic fever
En
12
Yellow fever
Most cases
520
Bunyaviridae
Bunyamwera
En
Germiston
En
Sand fly fever
Ep
Rift Valley fever
Ep
En
15
California encephalitis
En
1
Crimean hemorrhagic fever
En
1520
a
In addition to the disease manifestations listed, most viruses in this table can cause mild febrile illness; some viruses are endemic (En) but
others cause occasional outbreaks or epidemics (Ep).
b
Frequency relates to the relative number of cases exhibiting encephalitis or HF relative to the total number of infections. This number can be
difficult to estimate, since only the most seriously ill (e.g., hospital patients in an epidemic) may be reported as infections. Mortality ≥ 10%
is highlighted in red.
c
Percent mortality is the percent of those with encephalitis who succumb.
d
Percent mortality is the percent of those with HF who succumb.
e
Mortality in children is at the high end of the range given.
f
Mortality from recent epidemics in the United States.
g
Mortality generally lower in children.
in the gut, which might be important for virus spread from
results in decreased survival and reproductive capacity.
the gut into the hemolymph, from which it spreads to the
Thus, the persistence of an arbovirus requires a delicate
salivary glands, in some cases after an increase in titer
balance. The virus must replicate to sufficiently high titer
following replication in fat body cells. More importantly,
in the vertebrate to cause sufficient viremia to infect a
however, are studies showing that arbovirus infection
mosquito, which usually results in symptomatic disease.
But in the mosquito the infection must be controlled so
Seasonality of Disease
as to produce sufficiently high titers of virus in salivary
It is obvious from the preceding discussion that mos-
fluid without damaging too extensively the ability of the
quito-borne diseases are seasonal. In temperate areas, dis-
mosquito to survive and reproduce.
ease is absent in the winter. In spring when mosquitoes
first arise, disease is also absent or rare. It is only with
Overwintering by Arboviruses
time that the intensity of virus transmission builds up to
In humid tropical or subtropical areas in which
the point that humans become infected with some fre-
mosquitoes are active throughout the year, an arbovirus
quency. There have also been suggestions that the arbo-
can be maintained by continuous transmission between
viruses that first appear may not be as virulent as viruses
invertebrate and vertebrate hosts. Virus activity may vary
that appear later in the season, perhaps because they are
during the year, for example it may be much greater dur-
adapted to the mosquito vector and must readapt to the
ing a rainy season when the number of mosquito vec-
vertebrate host. In any event, arboviral epidemics char-
tors is higher, but the virus is active throughout the year
acteristically occur in mid to late summer and early fall
and human infection can occur at any time. However, in
in temperate regions. In other regions, epidemics may be
temperate zones in which adult mosquitoes die off in the
associated with heavy rainfall that results in an increase
winter, or in very dry areas in which mosquitoes are only
in the mosquito population.
active after sporadic rains, the virus must have a mecha-
nism by which it overwinters. Mosquitoes survive win-
Encephalitic Alphaviruses
ters (or extended droughts) by suspending development
of the young at some stage. In some mosquitoes, eggs are
Most, perhaps all, alphaviruses are neurotropic. They
laid but do not develop until conditions are favorable. In
readily infect neurons in culture or in experimental ani-
others, developing young diapause, suspending develop-
mals. Infection of neurons is dependent upon the age of
ment at some stage of embryonic development or larval
the animal as well as dependent upon the strain of virus.
development, until conditions are favorable, such as the
Sindbis virus infection of the mouse has been used as
return of spring. Thus, one mechanism for overwintering
an experimental model to study virus induced encepha-
by some arboviruses is transovarial transmission, in which
litis (inflammation of the brain, from enceph = brain,
the virus infects oocytes in the infected female at an early
itis = inflammation) and encephalomyelitis (inflamma-
stage of development; the replication cycle of the virus is
tion of the brain and spinal cord, from the preceding plus
suspended during diapause and the animal develops nor-
myel = medulla or marrow). Some strains of the virus will
mally. When the newly hatched mosquito begins to fly, it is
invade the central nervous system after peripheral inocu-
already infected. One way to search for transovarial trans-
lation and cause encephalitis, whereas other strains of the
mission in the field is to look for virus in male mosquitoes.
virus do not invade the CNS following peripheral inocula-
Since these do not feed on blood, the only way for them
tion but will cause encephalitis upon direct inoculation of
to become infected is by transovarial transmission. Some
the virus into the brain, and still other strains do not cause
alphaviruses are known to use this mechanism whereas
overt encephalitis even though neurons may be infected.
other alphaviruses do not. A second mechanism used by
In all cases, the infection of neurons to cause encephalitis
some arboviruses is persistent infection of a vertebrate host
is age related. Very young mice are susceptible to most
so that infected vertebrates are present when the mosqui-
strains of the virus, whereas infection of older mice does
toes emerge once again. Some alphaviruses are known to
not result in encephalitis for many strains of the virus.
persist in humans or other vertebrates for extended periods,
Manipulation of the viral genome in the laboratory has
but such persistence is rare and it is not known whether this
resulted in the identification of specific genes that are
could be a means of overwintering. In some arboviruses,
important for neurovirulence.
however, such as the coltiviruses (see Chapter 5), persis-
It is important to distinguish neurotropism, the ability to
tence is known to be essential to the maintenance of the
infect neurons or other cells of the CNS, from neuroinvasion,
virus in nature and the virus has evolved specific mecha-
the ability of the virus to cross the bloodbrain barrier and
nisms to persist. A third mechanism is the reintroduction of
invade the CNS, from neurovirulence, the ability to cause
the virus into an area from regions where it persists year-
brain disease once the CNS has been invaded. Although
round, for example, by the return of migratory birds or by
most alphaviruses studied are neurotropic, only three viruses
infected mosquitoes being blown over large distances by
regularly cause encephalitis in humans or domestic animals.
storms. The whole subject of overwintering is an interest-
These three viruses, Eastern (EEE), Western (WEE), and
ing evolutionary and ecological study, and in the case of
Venezuelan equine encephalitis (VEE) viruses, are New
many arboviruses, including most alphaviruses, overwin-
World alphaviruses that cause fatal encephalitis in horses.
tering is only poorly understood.
WEE and EEE regularly cause encephalitis in humans as
Western Equine Encephalitis
13
27
78
42
1
2
40
1
14
5
26
1
6
17 3
36
7
53
Rate per 100,000 population
3
none
2
13
<0.01
or equine only
94
0.01 0.10
0.11 0.59
0.60 1.00
>1.0
Eastern Equine Encephalitis
1
1
20
2
12
4
2
19
1
3
3
4
11
9
1
23
2
6
1
7
55
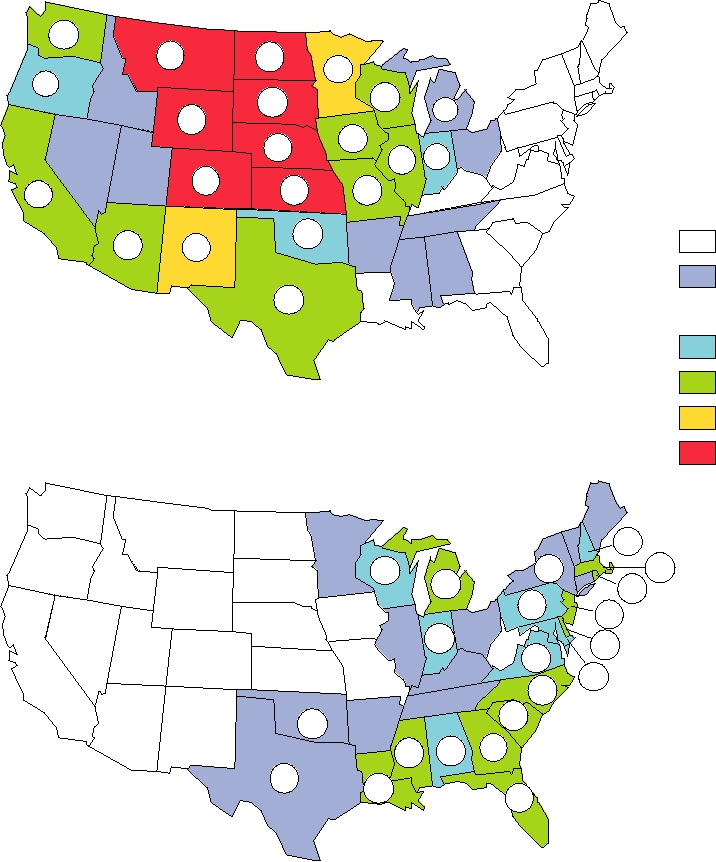
FIGURE 3.24 Geographic distribution of reported human cases of alphavirus encephalitis in the United States from 1964
to 2003. Colors indicate the rate in cases per 100,000 population by state and the actual numbers of cases are shown. Note that
there were two human cases of WEE in 1994 and one in 1999, but none have been reported since then. Reported cases of EEE
from 1994 through 1998, for which no state locations were given, were 1 in 1994, 4 in 1995, 5 in 1996, 14 in 1997, and 4 in
1998. There were 5 cases of EEE in 1999, 3 in 2000, 9 in 2001, 10 in 2002, and 14 in 2003. Since 1998 EEE cases are reported
by state and the cases for 19992003 have been added to the cumulative state totals. Adapted from Fields et al. (1996) p. 875
and additional data from MMWR, Summary of Notifiable Diseases, 1998, 1999, 2000, 2001, 2002, 2003.
well, although the number of cases is small (Table 3.11 and
may be important for the invasion of the CNS by poliovirus
Fig. 3.24). The mechanisms by which the CNS is invaded
(see earlier). Infection of the nasal neuroepithelium, whose
by alphaviruses or by viruses belonging to other families
neurons project directly to the CNS, may also lead in infec-
that cause encephalitis (poliovirus, certain flaviviruses, and
tion of the CNS. Sindbis virus appears to use this mechanism
others) are imperfectly known. Model studies in mice have
as well as transport from the peripheral sites, and transport
indicated that Sindbis virus invades the CNS by retrograde
from the neural epithelium may be particularly important
axonal transport from the peripheral site of infection where
in VEE infection. A third possible mechanism is the estab-
primary replication occurs, one of the mechanisms that also
lishment of viremia followed by infection of choroid plexus
epithelial cells such that the virus replicates across the
reservoir for the virus. Perhaps because of this, the virus has
bloodbrain barrier. As described, this mechanism appears to
evolved into a number of strains that differ by up to 25% in
be particularly important in poliovirus invasion of the CNS,
nucleotide sequence.
but does not appear to be used during invasion by Sindbis
Western Equine Encephalitis Virus
virus. Thus, the primary mechanism used appears to differ
among different viruses, and more than one mechanism may
WEE is less virulent for humans than is EEE. Encephalitis
be important for any individual virus.
occurs in only 1 of 1000 adults infected by WEE, but in
Alphaviruses have been used as a model system to study
about 2% of children younger than 5, and the encephalitis
recovery of mice from virally induced encephalitis. The CNS
produced by WEE is less severe, with an overall case fatality
is immunologically privileged (see Chapter 10) because
rate of about 3%, but about 8% in persons older than 50. The
neurons are not replaceable once destroyed, and the mecha-
virus is also less virulent in horses, in which the case fatal-
nisms used to cure the CNS of viruses differ from those used
ity rate is about 40%. The virus is a recombinant between
in other organs such as the liver or intestinal tract. It has been
EEE and a Sindbis-like virus, probably Aura virus present
shown that humeral antibodies are required for clearance
in South America (Fig. 3.17). Because the glycoproteins
from the CNS but the mechanism is unknown. Cytotoxic T
were derived from the Aura parent, the virus is serologically
cells are also required for recovery from virally caused CNS
related to the Sindbis lineage. The RNA replication proteins
disease, but by means of secretion of interferon-γ rather than
are derived from EEE, as is the encephalitic potential of the
by killing of infected cells.
virus. The fact that the virus is less virulent than EEE is con-
sistent with laboratory studies that chimerization of a virus, a
Eastern Equine Encephalitis Virus
technique being used to produce live virus vaccines, usually
The majority of human infections by EEE are inap-
results in lowered virulence (see, e.g., Chapter 11).
parent or result in febrile illness that is usually mild.
WEE is endemic from western Canada discontinuously
Encephalitis results in only about 4% of infected adults
to southern South America. In the western United States,
but in more than 10% of children less than 10 years old,
the primary vector is Culex tarsalis and the vertebrate reser-
demonstrating the age-related aspects of the disease.
voir is again birds, although jackrabbits and possibly other
EEE-caused encephalitis is fatal about half the time, with
mammals may be important in some areas. In the past,
the highest fatality rates in the very young and the very
infection by the virus was common. An epizootic of WEE
old. Survivors usually have neurological deficits. There
in 1912 killed an estimated 25,000 horses in the western
have been an average of seven cases of EEE encephalitis
United States. In 1960, 34% of humans tested in rural areas
per year in the United States over the last 50 years. Horses
of California were found to be positive for antibodies to
are more sensitive to the virus and more likely to become
WEE, indicating past infection by the virus. Infection is
infected, and the virus is of major concern to horse breed-
now less common in the United States, and only about 2%
ers in the eastern United States. The case fatality rate is
of humans in similar areas of California were found to be
8090%, and in years past there have been epidemics
seropositive for WEE in the mid 1990s. Confirming this
involving thousands of horses; the largest such epidemic
trend, an average of 34 cases of human WEE encephalitis
on record killed more than 11,000 horses in Louisiana and
occurred per year in the United States from 1955 to 1984,
Texas in 1947.
but this number has declined thereafter and there has been
As described before, in North America EEE is main-
only one case in the United States since 1994 (Fig. 3.24).
tained in an enzootic cycle in birds that inhabit freshwater
This dramatic decline may have resulted from mosquito
swamps, vectored by Culiseta melanura. Perhaps because
control measures, the widespread use of insect repellents,
many of these birds are migratory and therefore capable of
the adoption of air-conditioning and window screens that
spreading the virus over large distances, the virus is fairly
has resulted in fewer bites by mosquitoes, especially night
uniform throughout its North American range. Viruses iso-
flying mosquitoes, and because fewer horses, which are
lated over a period of more than 60 years from many differ-
amplifying hosts for WEE, are used in farming. No vaccines
ent areas of North America show less than 2% nucleotide
for this virus are available for widespread use, although
sequence divergence. As illustrated in Fig. 3.23, epizootic
experimental vaccines exist that are given to laboratory per-
cycles in other birds can occur. Mosquitoes vectoring either
sonnel who work with the virus.
the enzootic cycle or an epizootic cycle are capable of trans-
In Central and South America, WEE remains a widespread
mitting the disease to horses or humans.
problem because the horse is still in widespread use as a farm
EEE in South America is a distinct virus. It is vectored
animal. However, the virus is not associated with significant
primarily by Culex species and is not associated with severe
human disease in South America, for reasons unknown. In
disease in humans. In addition to birds, small mammals,
South America, small mammals appear to be an important
especially rodents, which are sedentary, are an important
vertebrate reservoir.
As described, WEE arose by recombination, an event that
in Fig. 3.25) is also a South American virus but is only dis-
occurred at some unknown time but probably more that 1000
tantly related to other subtype I viruses, and is now consid-
years ago. Since its origin, it has diverged into a number
ered a separate species, named to the present by its isolate
of different viruses, including Fort Morgan virus found
designation 78V353I virus. These eight virus species are
in Colorado and Oklahoma and Highlands J virus present
maintained in an endemic cycle in which the principal mos-
along the east coast of the United States. Fort Morgan virus
quito vectors are species belonging to the genus Culex, sub-
is transmitted by swallow bugs present in the nests of swal-
genus Melanoconin, and the major vertebrate reservoirs are
lows to the young birds of the year, an interesting adaptation.
small mammals, primarily cotton rats and opossums. These
Highlands J virus is maintained in a cycle similar to that
endemic viruses are able to infect horses but replicate to only
for EEE. Neither of these viruses has been associated with
low titers in them, causing no disease and not establishing
human disease.
an epidemic cycle. Similarly, these endemic viruses are not
associated with significant human disease.
Venezuelan Equine Encephalitis Virus Complex
The endemic VEE ID gives rise to epizootic viruses IAB
The VEE complex consists of a number of closely related
and IC by a small number of mutations in envelope glycopro-
viruses that are endemic to tropical and subtropical areas of
tein E2 that allow the virus to grow to high titers in horses.
the Americas. These viruses were first classified by serology
The horse serves as an important amplifying host that allows
into six subtypes of VEE, numbered with Roman numer-
the virus to become epidemic and spread over wide areas.
als from I to VI. Some of these subtypes were further sub-
Aedes taeniorhynchus and Psorophora confinnis are impor-
divided; in particular, subtype I was subdivided into IAB,
tant mosquito vectors in these outbreaks. In these epidem-
IC, ID, IE, and IF. Partial or complete sequences of most of
ics, which occur at intervals of 1020 years in Venezuela,
these viruses are now available and these subtypes are now
Columbia, Peru, and Ecuador, large numbers of horses die of
considered to be full species (Fig. 3.25). As a species, VEE
encephalitis and significant episodes of human illness occur.
virus consists of IAB, IC, and ID; IE, also considered a strain
For example, an epidemic in Venezuela and Columbia in
of VEE, will be considered in more detail later. Subtype II
1995 resulted in disease in an estimated 75,000 to 100,000
is now named Everglades virus and is endemic to Florida.
people, including 3000 cases of neurological disease and 300
Subtype IIIA is called Mucambo virus, IIIB is Tonate virus
deaths. As with the other encephalitic alphaviruses, children
(of which Bijou Bridge virus is another isolate), IV is Pixuna
and older people are more at risk for serious illness.
virus, V is Cabassou virus, and VI is Rio Negro virus; most
Because of the importance of VEE epidemics, an inac-
of these are South American viruses. Subtype IF (not shown
tivated virus vaccine was produced many years ago from a
Serological
Subtype
VI
Rio Negro
Pixuna
IV
Cabassou
V
VEE-71D1252
IIIC
Mucambo
IIIA
Tonate
IIIB
Bijou Bridge
VEE
IE
Everglades
II
VEE
ID
VEE
IAB
0.05
VEE
IC
Substitutions/site
FIGURE 3.25 Phylogenetic tree of the various strains of Venezuelan equine encephalitis virus (VEE) and species of
viruses formerly considered to be serological subtypes of VEE. This tree was generated from partial E1 envelope gene
sequences using a neighbor-joining algorithm. Adapted from Fauquet et al. (2005), Figure 4 on p. 1007.
strain of IAB virus called Trinidad Donkey for use in horses
annulirostris and Aedes notoscriptus in other areas, which
and for laboratory personnel who work with the virus. This
breed in freshwater. The vertebrate reservoir consists of var-
vaccine suffered from poor immunogenicity and insuffi-
ious macropods. Because mammals constitute the vertebrate
cient inactivation. Use of the vaccine, for example, led to
reservoir, different strains of the virus have evolved in differ-
a wide-ranging epidemic of VEE in 19691973 that spread
ent geographic regions for reasons discussed earlier. In some
from South America up through Central America to south-
areas of Australia in which the virus is endemic, a majority
ern Texas, causing many thousands of cases of disease in
of the population may be seropositive for RRV, indicating
horses and humans. Because of these problems, use of this
a high attack rate. It has been estimated that 2 to 30% of
vaccine was abandoned and a new attenuated vaccine strain
infected humans develop clinical symptoms following infec-
called TC-83, derived from the Trinidad Donkey virus by
tion. As noted, recovery may be prolonged, with arthritic
passage in culture, was developed. This vaccine is effective
symptoms recurring over a period of a year or more.
but is reactogenic and causes mild illness in many recipients.
Of interest was a wide-ranging epidemic of Ross River
An inactivated version of this attenuated virus has also been
polyarthritis that swept through the South Pacific in 19791980.
produced as a vaccine but is not as effective as the live virus
The epidemic began when a single viremic traveler from
vaccine.
Australia landed in Fiji. The epidemic began near the airport
VEE virus can spread by aerosols as well as by mosquito
and eventually spread throughout the island. From there it
transmission. Many cases of laboratory acquired infection
jumped to other islands having air contact with Fiji. During
have resulted from aerosols. Because of this property and the
this epidemic, it is believed that humans were the primary
incapacitating disease caused by VEE, this virus was weap-
or only vertebrate host, with the disease being transmitted
onized by the Russians in the past as a potential biowarfare
from mosquito to human to mosquito without the interven-
agent.
tion of another animal reservoir (the cycle illustrated in Fig.
Virtually all epidemics of epizootic VEE have been due
3.23A). Sequencing studies have suggested that a mutation
to IAB and IC viruses. However, a recent epizootic of VEE
in glycoprotein E2 may have allowed this cycle to become
in Mexico resulted from an IE strain that had a mutation in
established. The mosquito vector during this epidemic was
envelope glycoprotein E2. Unlike the IAB and IC strains,
Aedes polynesiensis. This epidemic was explosive with most
the epizootic IE virus grows to only low titers in horses. It is
of the people on the affected islands becoming infected by
believed that the mutation in IE virus allowed a more effi-
the virus and about 10% of them becoming ill. The epidemic
cient interaction with mosquito vectors that are widespread
eventually burned itself out because all the humans had
and numerous, allowing more efficient transmission of the
become immune and the virus failed to establish an endemic
virus. As shown in Fig. 3.25, IE virus is more closely related
cycle in other animals in the region. After 20 years without
to Everglades virus than it is to ID virus, and the virus may
RRV in Fiji, three recent cases of RRV disease have been
be reclassified in the future.
reported in travelers to Fiji. It is thought that RRV has been
reintroduced into the island on these occasions and sus-
ceptible tourists infected, but that the native population is
Alphaviruses That Cause Arthritides
now largely immune to the virus and no new epidemic has
A number of alphaviruses cause disease in humans char-
occurred.
acterized by fever, rash, and joint involvement. The pain
Sindbis Virus
from arthritis (joint inflammation, from arth = joint and
itis = inflammation) or arthralgia (joint pain, from arth and
The prototype alphavirus is Sindbis virus, named after
algia = pain) following infection by these viruses can be so
the town of Sindbis, Egypt, where it was first isolated in
severe as to be disabling and can last for a year or more with
1953 from mosquitoes. It has the widest distribution of
relapses of severe arthritis being common during this period.
any alphavirus, endemic from northern Europe through
The names of some of these viruses come from the crippling
the Middle East and India to South Africa, Southeast Asia,
pain caused by the disease resulting from viral infection.
Indonesia, the Philippines, New Guinea, and Australia. As
might be expected from this broad range, strains of virus
Ross River Virus
isolated from different regions may differ by 20% or so in
Ross River virus is widespread in Australia, New Guinea,
nucleotide sequence and differ in their epidemiology and
and the Solomon Islands. The disease induced by virus infec-
disease potential. As far as known, birds are the vertebrate
tion is known as epidemic polyarthritis and is characterized
reservoir for the virus over its entire range with different
by pain, often accompanied by frank swelling, in the small
mosquitoes serving as vectors in different areas. Over most
joints of the hands and feet and in the knees. The princi-
of its range no human illness is associated with infection or
pal vectors are Aedes camptorhynchus and Aedes vigilax
illness is very rare, even though in regions such as the Nile
in coastal regions, which breed in salt marshes, and Culex
Delta seroprevalence rates may be fairly high. However,
strains of the virus in northern Europe, South Africa, and
In 2006, a hugh epidemic of CHIK, accompanied in many
Australia cause significant episodes of arthritic illness.
locations by infections of dengue virus as well (see later)
In northern Europe the strains of Sindbis virus that cause
whose disease symptoms can be similar to those caused by
arthritic disease are called Ockelbo virus in Sweden (present
CHIK, occurred throughout the Indian Ocean region. The
between the 60th and 64th parallels), Pogosta virus, wide-
virus appears to have been imported from East Africa, and
spread in Finland, and Karelian Fever virus, present in far
many thousands of cases occurred in the region, affecting
Western Russia. The virus is maintained in migratory birds
the islands of Reunion, Mauritius, Madagascar, Mayotte, the
or in game birds and the mosquito vectors are various spe-
Seychelles, and the Maldives as well as the Indian subconti-
cies of Culex and Culiseta. Aedes species may spread the
nent. In Reunion there occurred more than 200,000 cases in a
virus to humans. The disease in humans is typical of alphavi-
population of under 800,000. Many of these islands are pop-
rus arthritic disease with fever, rash, and joint inflammation
ular tourist destinations, and many cases were imported into
and the number of cases can be large during epidemic years.
Europe. The primary mosquito vector was Aedes albopic-
Children are less likely to develop disease upon infection.
tus or Aedes aegypti, depending upon the location, both of
In South Africa strains of Sindbis virus also cause human
which are also efficient vectors for dengue virus.
disease, but the number of cases appears to be small and the
O'nyong-nyong (ONN) virus is a close relative of CHIK
virus has been less well studied. Strains of Sindbis virus that
that has caused very large epidemics of disease in East
are present in Australia also cause human disease, primarily
Africa similar to that caused by CHIK. The name also comes
in northeastern Australia. The virus appears to be fairly uniform
from the very painful arthralgia accompanying the disease.
throughout Australia but to be replaced every so often by
Epidemics affecting up to two million people have occurred
new strains that presumably invade from the north.
followed by the disappearance of the virus for long periods.
In these epidemics the virus is transmitted by Anopheles
Mayaro Virus
funestrus and Anopheles gambiae, mosquitoes that are
Mayaro virus is present in the northern half of South
major vectors of malaria, and these epidemics represent the
America (Trinidad, Surinam, Brazil, Columbia, Bolivia). It
only known cases of epidemic transmission of an alphavi-
is maintained by Haemagogus mosquitoes and humans usu-
rus by anopheline mosquitoes. An endemic cycle presum-
ally contract the virus while in humid tropical forests. Rubber
ably maintains the virus during interepidemic periods but, if
workers are at risk of infection and the polyarthritis caused
so, this cycle is unknown. A strain of ONN called Igbo-Ora
by the disease can be debilitating, preventing the workers
virus, from the name of the Nigerian village in which the
from gainful employment. Mayaro belongs to the Semliki
virus was first isolated, is present in West Africa.
Forest virus clade (Fig. 3.17) and causes a disease that is
Barmah Forest Virus
similar to that caused by the related Ross River virus. It is
the only known representative of this clade in the Americas
Barmah Forest virus is an Australian virus that is an out-
and represents one of the very few transfers of alphaviruses
lier in the Semliki Forest clade (Fig. 3.17). It also causes
between the Old and the New World.
polyarthritis in humans. It is probably maintained in a cycle
similar to that for Ross River virus.
Chikungunya Virus and Related Viruses
Chikungunya (CHIK) virus, a member of the Semliki
Other Alphaviruses
Forest virus clade, is endemic or epidemic from sub-Saharan
Africa through India and Southeast Asia to the Philippines.
Other alphaviruses are known that infect higher verte-
The name comes from Swahili meaning "that which bends
brates including humans, but most are not associated with
up," from the intense arthralgia that causes patients to lie with
disease in humans. Semliki Forest virus, named after the
joints flexed. In Africa the virus is maintained in an endemic
Semliki Forest in Uganda, has been extensively charac-
cycle that is similar to that for yellow fever virus (see later).
terized as a model system to study the molecular biology
The mosquito vectors are Aedes africanus and Aedes furcifer
of alphaviruses. Most strains cause no human illness, but
and subhuman primates are the vertebrate reservoir. During
strains from central Africa cause a disease characterized by
explosive epidemics in urban areas of Africa and Asia, Aedes
exceptionally severe headache, fever, and rash. One case of
aegypti is the vector and humanmosquitohuman cycles
fatal human encephalitis caused by this virus occurred in a
maintain the virus. During an epidemic, a large fraction of
laboratory worker, who is believed to have contracted the
the susceptible human population may contract the disease.
virus via aerosols. Getah virus, widespread in Asia, causes a
The epidemic then dies out, to return when reintroduced
mild febrile illness in humans.
after a period of time that allows a susceptible population
Recent isolates of new alphaviruses include Southern
to build up. Epidemics often occur during the rainy season
elephant seal virus, currently unclassified. It is spread by
when the population of Aedes aegypti is highest.
a louse, Lepidophthirus macrorhini, that infests the seals.
Two fish alphaviruses have been recently isolated, salmon
synthesis is controlled in the same way as in alphaviruses.
pancreas disease virus and sleeping disease virus, which
The uncleaved nonstructural polyprotein synthesizes minus-
are now considered to be strains of the same species, to
strand RNA, whereas the cleaved products synthesize only
be called salmonid alphavirus. A third strain of the virus,
plus-strand RNA.
Norwegian salmonid alphavirus, has been found in west-
Rubella virus infects only humans and there is no other
ern Norway. Salmonid alphavirus is an important pathogen
reservoir. Infection is by person-to-person contact, primarily
of Atlantic salmon and rainbow trout in Norway, Britain,
through aerosols. It causes a relatively benign illness, some-
Ireland, and France. It causes significant economic losses
times called German measles, with a characteristic rash and
in the farmed fish industry and is the leading cause of eco-
is (or was) one of the typical childhood diseases. However,
nomic loss in Ireland. The viruses can be spread by direct
infection of a pregnant woman in the first trimester of preg-
fish-to-fish infection and are not known to have an arthro-
nancy can have devastating effects on the developing fetus.
pod vector, but since all other alphaviruses are vectored
The virus sets up a long-lived infection in the fetus that often
by arthropods there has been speculation that a sea louse
causes developmental abnormalities resulting in children
Lepeophtheirus salmonis might be involved in the transmis-
with severe handicaps (congenital rubella syndrome). An
sion of the virus. It is not known if the primary location of
attenuated virus vaccine against rubella has been developed
infection is saltwater or freshwater.
that is now routinely given to children as part of mumps
measlesrubella vaccination (MMR). Since the vaccine was
introduced, there has been a drastic reduction in the number
Rubella Virus
of cases of rubella in the United States (Fig. 3.26) and other
Rubella virus is less well understood than alphaviruses
developed countries.
because it grows very poorly in cultured cells and its genome
Because the postnatal disease caused by rubella virus is
possesses an extraordinarily high GC content (70%), which
trivial, the purpose of the rubella vaccine is to protect against
retarded efforts to sequence and express the viral genome.
future birth defects rather than to protect the individual vac-
The complete sequences of several strains are now known
cinated. This raises interesting ethical questions about its
and detailed molecular studies are under way. The genome
use. In some societies, only females were vaccinated, since
is approximately 10 kb in size and is expressed similarly to
they would want to protect their future children from the
the alphavirus genome: The genomic RNA is translated into
effects of rubella. However, because males remained suscep-
a polyprotein cleaved by a papain-like protease into two
tible to the virus, it continued to circulate in the population
pieces, and a subgenomic mRNA is translated into structural
and rubella-caused birth defects continued to occur. To pro-
proteins consisting of a capsid protein and two envelope
tect against this, the only solution is to vaccinate the entire
glycoproteins (Fig. 3.16). Interestingly, minus-strand RNA
population so as to eliminate the virus from the society.
Vaccine Licensed
35
30
1.0
0.8
25
0.6
0.4
20
0.2
0.0
15
1982
1987
1992
1997
2002
Year
10
5
0
1967
1972
1977
1982
1987
1992
1997
2002
Year
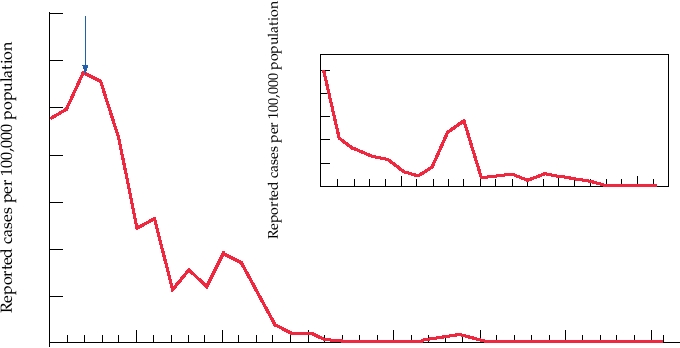
FIGURE 3.26 Incidence of rubella virus, by year, in the United States. From MMWR Summary of Notifiable Diseases in
the United States for 1997, 1998, 1999, 2000, 2001, 2002, 2003.
Search WWH :