Introduction
Over the past six decades, tourism has experienced continued expansion and diversification becoming one of the largest and fastest growing economic sectors in the world. Many new destinations have emerged alongside the traditional ones of Europe and North America. In the next years an increase of travelling is expected, and the number of related infections will also be higher (http://www.unwto.org/facts/menu.html). Rickettsioses are an important topic in the field of travel medicine.
Rickettsiae are small gram-negative intracellular bacteria (belonging to the alpha-1 proteobacteria) mainly transmitted by arthropods (lice, fleas, ticks and other acari) with two genera: Orientia with a unique specie (Orientia tsutsugamushi) and Rickettsia with several species. The clinical pictures that they cause are named rickettsioses (Raoult, 2010a). Rickettsioses have been a threat all along the History and nowadays they are an important cause of morbi-mortality in some areas of the world. To know the distribution of the different diseases caused by these bacteria and how the clinical pictures are recognized may be essential for a quick diagnoses and starting the correct treatment. Some of these infections can be also easily prevented with basic rules. Main rickettsioses with their distribution area are showed in the table 1.
In the 21st Century in most parts of the world hygienic conditions have improved and epidemic typhus is absent. To acquire this condition it is necessary to be in contact with body lice. Furthermore, if people have personal hygiene and change their clothing, body lice are removed. Nevertheless it is possible that if we travel for cooperation to catastrophic areas or other places with poverty, we may take body lice (refugees’ camps) and may develop exanthematic typhus.
There are a lot of references of rickettsioses acquired by travellers and considered imported diseases (McDonald et al., 1988; Bottieau et al. 2006; Freedman et al., 2006; Askling et al. 2009; Chen & Wilson, 2009; Jensenius et al., 2009; Stokes & Walters, 2009). Nowadays ticks cause most travel-associated rickettsioses. Ticks are considered to be one of the most important vectors of infectious diseases in the world, preceded only by mosquitoes. Therefore, tick-borne rickettsioses are endemic all over the world (Hechemy et al., 2006). The majority of travel-associated rickettsioses refer to Sub-Saharan Africa tourists who develop African tick-bite fever (ATBF), mainly transmitted by Amblyomma hebraeum (Figure 1). In addition to malaria, ATBF is an important cause of fever in people returning from the tropic (Field et al., 2010). Other reports describe Mediterranean spotted fever (MSF) acquired by tourists bitten by Rhipicephalus spp. ticks (Figure 2) when visiting Europe, being more scarce references about other rickettsioses. Flea-borne rickettsioses and chigger-transmitted rickettsioses are less frequent in travellers and tourists, and some of them as murine typhus are associated with poor hygienic conditions. Most travel-acquired rickettsioses are related to outdoors leisure activities, like camping, trekking, hunting, safaris, etc.
It will be impossible to describe all rickettsioses in few pages. Since rickettsioses have very similar clinical pictures and they can be grouped in different syndromes, we will describe these syndromes emphasizing the typical features (i.e.: Presence of eschar or type of rash). Afterwards, distribution can be observed in the table 1. We will also write a specific paragraph for some infections (i.e.: Diseases caused by Rickettsia akari and Orientia tsutsugamushi).
Fig. 1. Amblyomma hebraeum, the principal vector of African-tick bite fever (ATBF) in southern Africa.
Fig. 2. Rhipicephalus spp., the main vector of Mediterranean spotted fever (MSF).
|
DISEASE |
CAUSATIVE AGENT |
VECTOR |
DISTRIBUTION |
|
Epidemic typhus |
R. prowazekii |
Body lice (Pediculus humanus corporis) |
Peru, northern Africa, Senegal, Burundi, Rwanda, Russia, sporadic cases in USA associated with flying squirrels. Potentially, all over the world associated to poverty and dirt. |
|
Murine typhus |
R. typhi |
Fleas (Xenopsylla cheopis and Ctenocephalides felis) |
All over the world (more prevalent in tropical and subtropical areas) |
|
Flea-borne spotted fever |
R. felis |
Cat fleas (Ctenocephalides felis) |
All over the world |
|
DISEASE |
CAUSATIVE AGENT |
VECTOR |
DISTRIBUTION |
|
Scrub typhus |
Orientia tsutsugamushi |
Trombiculid mite larvae (chiggers) |
Thailand, Laos, India, Pakistan, Kashmir, Sri-Lanka, Afghanistan, Nepal, China, Japan, Korea, Indonesia. Philippines, Papua-New Guinea, Australia |
|
Rickettsialpox |
R. akari |
Mouse mites (Liponyssoides sanguineus) |
Eastern Europe, Korea, South Africa, USA |
|
RMSF1 |
R. rickettsii |
Dermacentor variabilis and other American ticks |
USA, Mexico, Colombia, Brazil, Argentina, Panama, Costa Rica |
|
RMSF-like |
R. parkeri |
Amblyomma spp. ticks |
USA, Uruguay, Brazil, Argentina |
|
MSF2 |
R. conorii conorii, R. conorii israelensis, R. conorii caspia, R. conorii indica |
Rhipicephalus spp. ticks |
Mediterranean area, Central Europe, Russia, India and Africa |
|
MSF-like |
R. monacensis |
Ixodes ricinus ticks |
Europe |
|
MSF-like |
R. massiliae |
Rhipicephalus sanguineus ticks |
Mediterranean area, Argentina, USA? |
|
MSF-like |
R. aeschlimannii |
Hyalomma marginatum ticks |
Africa, Europe? |
|
DEBONEL / TIBOLA2 |
R. slovaca R. rioja R. raoultii |
Dermacentor marginatus ticks |
Europe |
|
LAR4 |
R. sibirica mongolitimonae |
Hyalomma spp. and Rhipicephalus pusillus ticks |
Europe, Africa. |
|
ATBF5 |
R. africae |
Amblyomma spp. ticks |
Sub-Saharan Africa and West Indies |
|
Siberian tick typhus |
R. sibirica sibirica |
Dermacentor spp. ticks |
Russia, Pakistan, China |
|
R. helvetica infection |
R. helvetica |
Ixodes ricinus ticks |
Central and northern Europe, Asia |
|
Japanese spotted fever |
R. japonica |
Ticks |
Japan |
|
Queensland tick typhus |
R. australis |
Ixodes spp. ticks |
Eastern Australia |
|
Flinder’s Island spotted fever |
R. honei |
Ticks |
Australia, Southeast Asia |
|
Far Eastern spotted fever |
R. heilonjgiangensis |
Dermacentor spp. ticks |
Eastern Asia |
!RMSF: Rocky Mountain spotted fever; 2MSF: Mediterranean spotted fever; 3DEBONEL/TIBOLA: Dermacentor-borne, necrosis, erythema, lymphadenopathy/Tick-borne lymphadenopathy; 4LAR: Lymphangitis-associated rickettsiosis; 5ATBF: African tick-bite fever.
Table 1. Rickettsia spp. causing medical diseases, vectors and distribution
Typhus syndrome
Typhus syndrome refers to a febrile syndrome with mental status impairment and rash. It is caused by Rickettsia prowazekii (epidemic typhus) and Rickettsia typhi (formerly, R. mooseri). Rickettsia felis may also produce a typhus syndrome named flea-borne spotted fever, which is similar to R. typhi infection (perhaps less severe) (Walker & Raoult, 2010; Dumler & Walker 2010; Oteo et al., 2006).
Nowadays epidemic typhus is only present in some regions of Africa, Russia and in Peru. It is associated with bad hygienic conditions that are necessary for body lice parasitization. Sporadic cases associated with contact with flying squirrels and their parasite arthropods, which have been involved as new reservoirs of the infection, have also been reported in USA. A possible source of R. prowazekii infection may be a recrudescent case (Brill-Zinsser disease) of R. prowazekii infection. If hygienic conditions are altered and an epidemic of body lice appears may be an epidemic of typhus, as occurred in Burundi with hundreds of affected people (Raoult et al., 1998). Some cases of louse-borne typhus in travellers have been published (Zanetti et al., 1998; Kelly et al., 2002).
Endemic typhus or murine typhus is associated with the presence of fleas. The main vector is the rat flea (Xenopsylla cheopis) associated with dirt and poor hygienic conditions. Flea-borne spotted fever is associated with the cat flea, and in this case bad hygienic conditions are not necessary. Murine typhus and flea-borne spotted fever are distributed all over the world. Although they are more frequent in tropical and subtropical areas, cases have also been reported in the Mediterranean area (Greece, Italy, Spain, France and Portugal) (Bernabeu-Wittel et al., 1999; Angel-Moreno et al., 2006; Gikas et al,. 2009; Pérez-Arellano et al., 2005; Oteo et al., 2006).
The clinical pictures of murine typhus and flea-borne spotted fever are less severe than the one of epidemic typhus. Thus, 1-2 weeks after the flea exposure, patients begin with fever, headache, myalgia, nausea and vomiting. Rash can be difficult to see in some cases, but is present until 80%. For R. typhi infection, rash is macular or maculo-papular and typically affects trunk and less frequently extremities. In epidemic typhus, petechial rash is more frequent than in endemic typhus, and cough, nausea and vomiting are frequent features. On the contrary of tick-borne rickettsioses or scrub typhus, an inoculation eschar (tache noire) is not observed. In most cases, fever and rash disappear in a few weeks but complications can be developed (central nervous, kidney involvement with renal insufficiency, respiratory failure, etc.). These complications are more frequent for epidemic typhus and in older people or patients suffering chronic diseases (Walker & Raoult, 2010; Dumler & Walker 2010). In all these conditions a raise in hepatic enzymes, C reactive protein as well as in leucocytes and platelets counts can be observed. We can also observe hepatosplenomegaly. In severe cases mainly associated with epidemic typhus, evolution to a multiple-organ dysfunction syndrome and coagulation disorders may appear.
Some references related to travellers are: Zanetti et al., 1998; Niang et al., 1999; Kelly et al., 2002; Jensenius et al., 2004; Azuma et al., 2006; Angelakis et al., 2010; Walter et al., 2011.
Tick-borne spotted fever
Tick-borne spotted fever are worldwide distributed and the clinical picture is very similar, although the severity is different related with the Rickettsia species involved.
ATBF and MSF are the most frequent tick-borne spotted fever rickettsioses in travellers (Smoak et al., 1996; Fournier et al., 1998; Oteo et al., 2004a; Raoult et al., 2001; Caruso et al., 2002; Jensenius et al., 2003; Roch et al., 2008; Tsai et al., 2008; Consigny et al., 2009; Stephany et al., 2009; Althaus et al., 2010; Jensenius et al., 2004; Boillat et al., 2008; Laurent et al., 2009). For this reason, we will refer to these conditions taking into account that few differences in the incubation period and severity may exist. For Rocky Mountain spotted fever (RMSF) and MSF caused by R. conorii israelensis, higher mortality than with the rest of spotted fever rickettsioses has been communicated (de Sousa et al., 2003). Some features of the main spotted fever rickettsioses are shown in table 2.
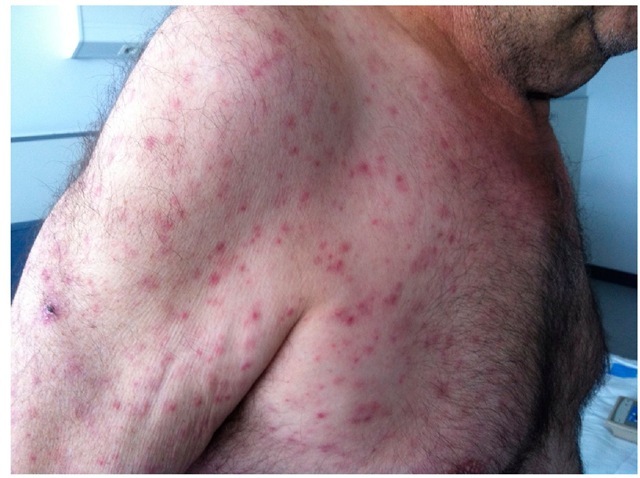
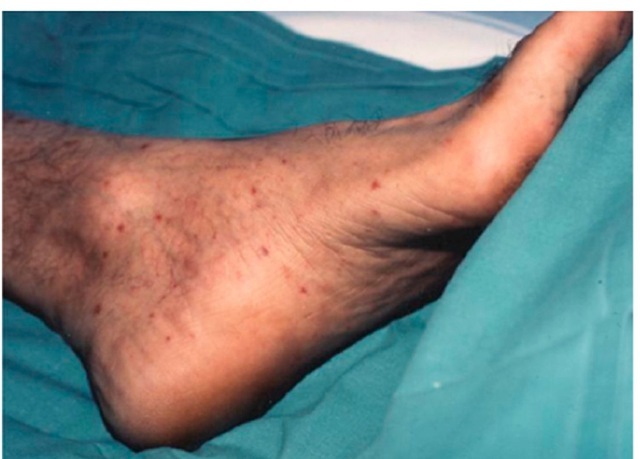
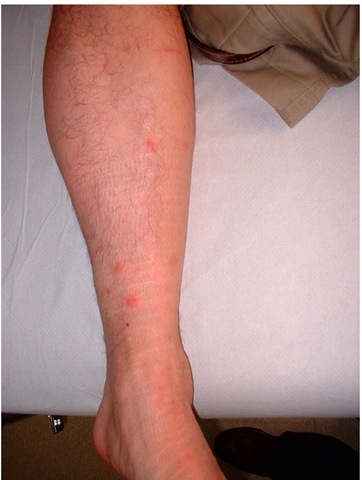
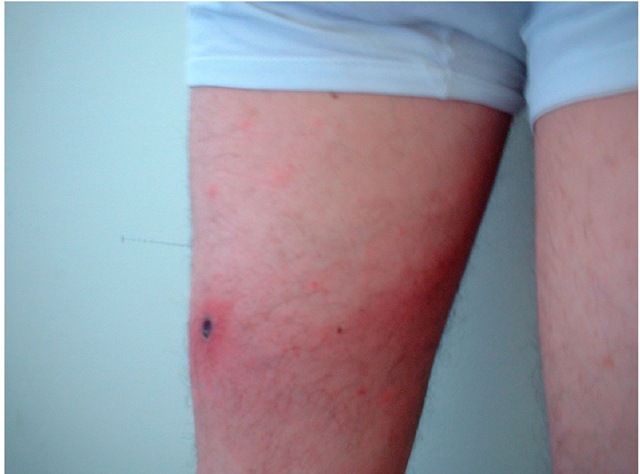
From 4 to 21 days after the tick bite, fever suddenly starts in 100% cases (less severe in ATBF). A characteristic inoculation lesion (eschar) (figure 3) is typically found until 72% of MSF cases and until 95% for ATBF. Multiple eschars are observed in some cases. This is more frequent in ATBF. Fever is accompanied of chills, headache, etc. (table 2). From 3 to 5 days after the onset of fever, the rash appears. This is a maculo-papular rash with purpuric elements in some cases (figure 4). It is more frequent in extremities and typically affects palms and soles. In ATBF the rash can be vesicular (figure 5), as occurs in R. akari and R. australis infections. For R. sibirica mongolitimonae infection, known as lymphangitis-associated rickettsiosis (LAR), lymphangitis from the eschar may appear in approximately 50% cases. It also can be observed in ATBF (Figure 6).
Fig. 3. Eschar (tache noire) and maculo-papular rash in a patient with Mediterranean spotted fever.
There are few reported cases of tickborne spotted fever caused by other of Rickettsia species (R. monacensis, R. aeschlimannii, R. massiliae, R. helvetica, R. sibirica mongolitimonae, R. parkeri, R. japonica and R. honei, among others) but it seems that the clinical pictures are very similar to the one of MSF cases. Data about the incidence of these infections among travellers to endemic areas are very scarce (Jensenius et al., 2004; Socolovschi et al., 2010).
For R. helvetica infections rash can be absent and fever is often the unique clinical manifestation. All these diseases are more frequent in spring and summer, when the vectors are more active. In all these conditions a raise in hepatic enzymes, C reactive protein and in leucocytes and platelets counts can be observed. We can also observe hepatosplenomegaly. In severe cases mainly associated to RMSF or MSF, evolution to a multiple-organ dysfunction syndrome and coagulation disorders may appear.
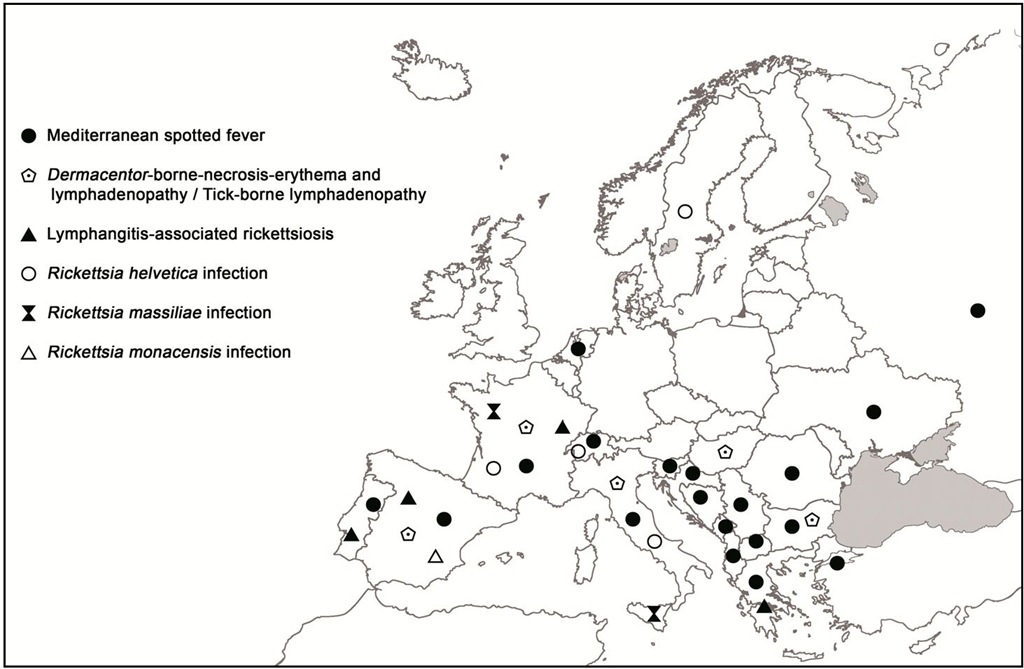
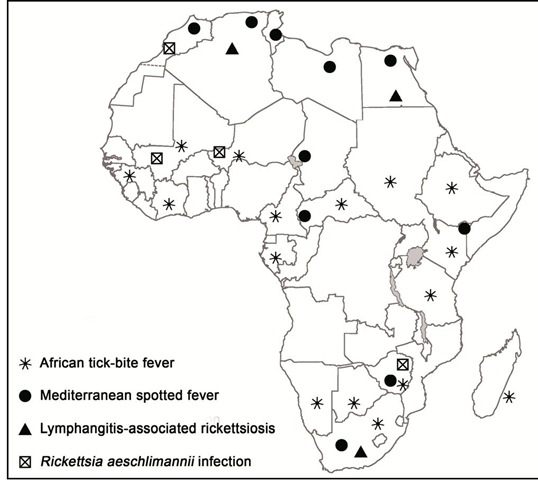
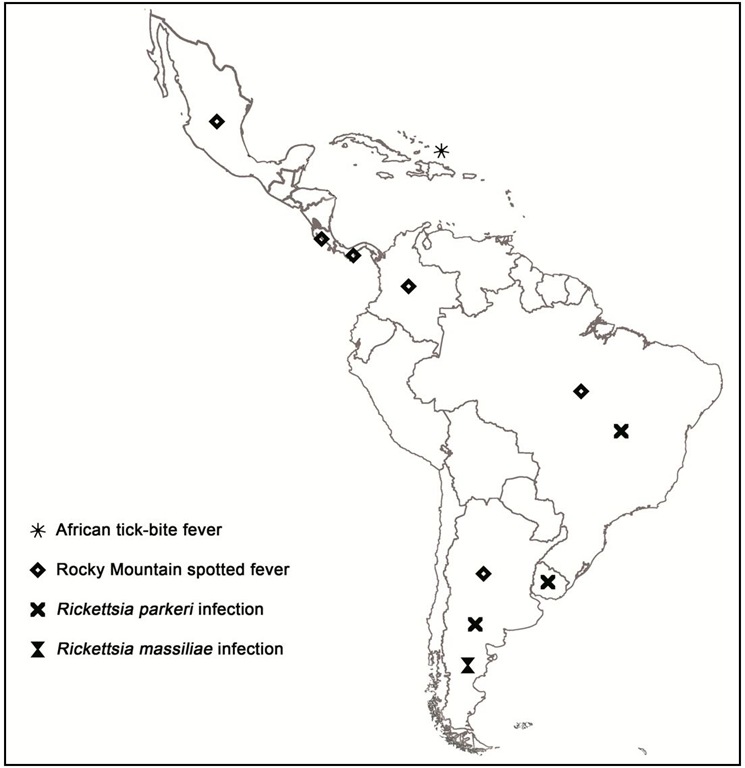
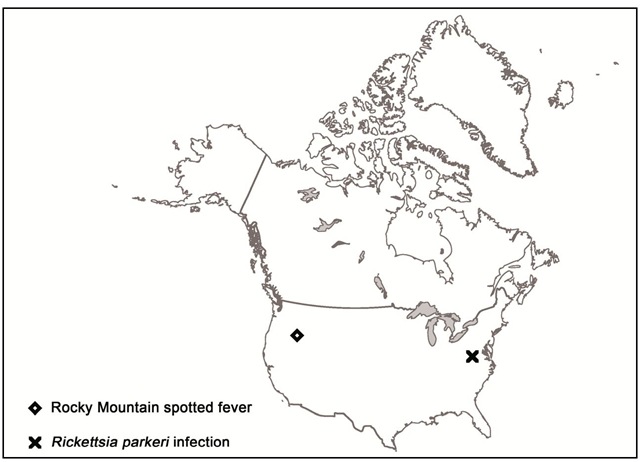
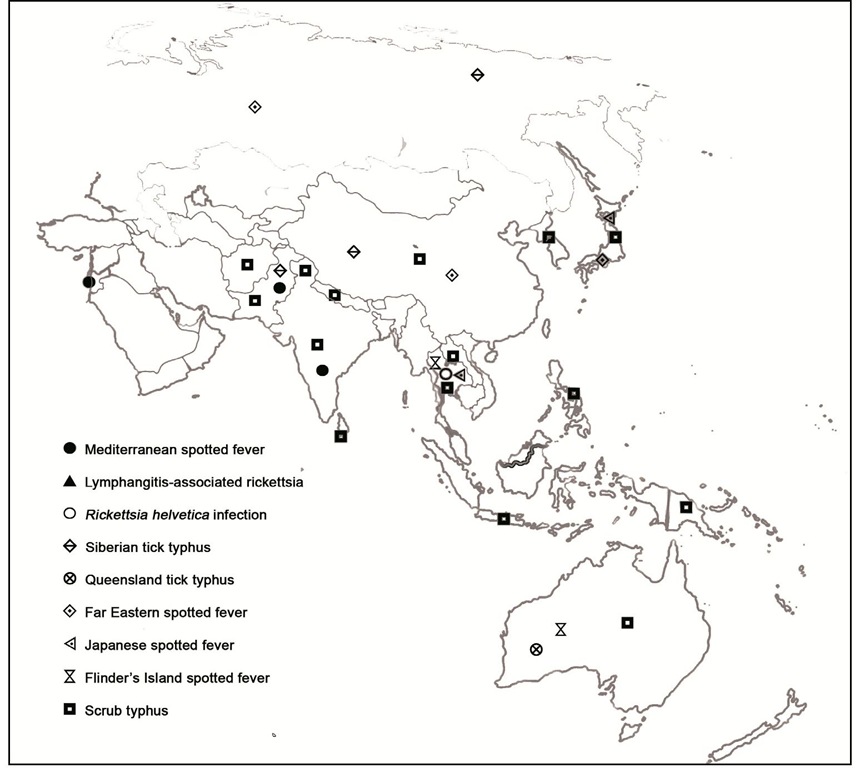
Distribution of human cases of tick-borne rickettsioses in Europe, Africa and Americas are showed in figures 7-10. Human cases of tick-borne rickettsioses and scrub typhus in Asia and Oceania are showed in figure 11.
|
DISEASE |
RASH |
SPECIFITIES |
ESCHAR |
FEVER |
|
MSF1 |
>95% |
10% purpuric rash |
72%. Multiple in 32% (children) |
100% |
|
RMSF2 |
90% |
45% purpuric rash |
<1% |
100% |
|
ATBF3 |
30% |
Vesicular rash |
100%. Frequently multiple |
100% |
|
DEBONEL/TIBOLA4 |
Possible |
Lymph nodes |
100%. Larger than in other rickettsioses |
30% |
|
LAR5 |
>90% |
50% lymphangitis |
Frequent |
100% |
|
R. aeschlimannii infection |
Possible |
- |
Possible |
100% |
|
R. helvetica infection |
Possible |
- |
Absent |
Not always |
|
R. massilliae infection |
Possible |
Rash can be purpuric |
Possible |
100% |
|
R. monacensis infection |
Possible |
- |
? |
100% |
|
Queensland tick typhus |
100% |
Vesicular rash |
65% |
100% |
|
Flinder’s Island spotted fever |
85% |
8% purpuric rash |
28% |
100% |
|
Siberian tick typhus |
100% |
- |
77% |
100% |
|
Japanese spotted fever |
100% |
- |
90% |
100% |
|
Far eastern spotted fever |
Possible |
- |
Possible |
100% |
JMSF: Mediterranean spotted fever; 2RMSF: Rocky Mountain spotted fever; 3ATBF: African tick-bite fever; 4DEBONEL/TIBOLA: Dermacentor-borne, necrosis, erythema, lymphadenopathy/Tick-borne lymphadenopathy; 5LAR: Lymphangitis-associated rickettsiosis.
Table 2. Main clinical characteristics of tick-borne rickettsioses
Fig. 4. Vasculitic rash affecting soles in a patient with Mediterranean spotted fever.
Fig. 5. Vesicular rash in a patient with African-tick bite fever.
Fig. 6. Eschar and lymphangitis in a patient with African tick-bite fever.
Fig. 7. Map showing distribution of human cases of tick-borne rickettsioses in Europe.
Fig. 8. Map showing distribution of human cases of tick-borne rickettsioses in Africa.
Fig. 9. Map showing distribution of human cases of tick-borne rickettsioses in Latin America.
Fig. 10. Map showing distribution of human cases of tick-borne rickettsioses in North America.
Fig. 11. Map showing distribution of human cases of tick-borne rickettsioses and srub typhus in Asia and Oceania.