Strategies that make LAMP simple and cost-effective
Conventional LAMP reagents are supplied in liquid form, and they have to be stored below -20 °C, similar to most PCR reagents. However, because of the lack of a freezer and cold chain transportation system in most of the peripheral laboratories in developing countries, it is essential to formulate LAMP reagents, which can be stably preserved at ambient temperatures (Jorgensen et al., 2006; Aziah et al., 2007). The newly formulated LAMP reagents are dried down into the lid of the reaction tubes, thus obtaining preservation stability at ambient temperatures for more than 12 months. The dried LAMP reagents can be reconstituted quite easily by shaking the tubes after the addition of the purified DNA solution. Because the LAMP reagent for each reaction is deposited on the individual tubes in advance, there is no longer a need to prepare and dispense master-mix solutions to the reaction tubes. Thus, liquid handling using micropipette, one of the most skillful steps, becomes unnecessary in the course of the assay. Moreover, this can contribute to reduced risk of carryover contamination during the assay.
Detection – Calcein method
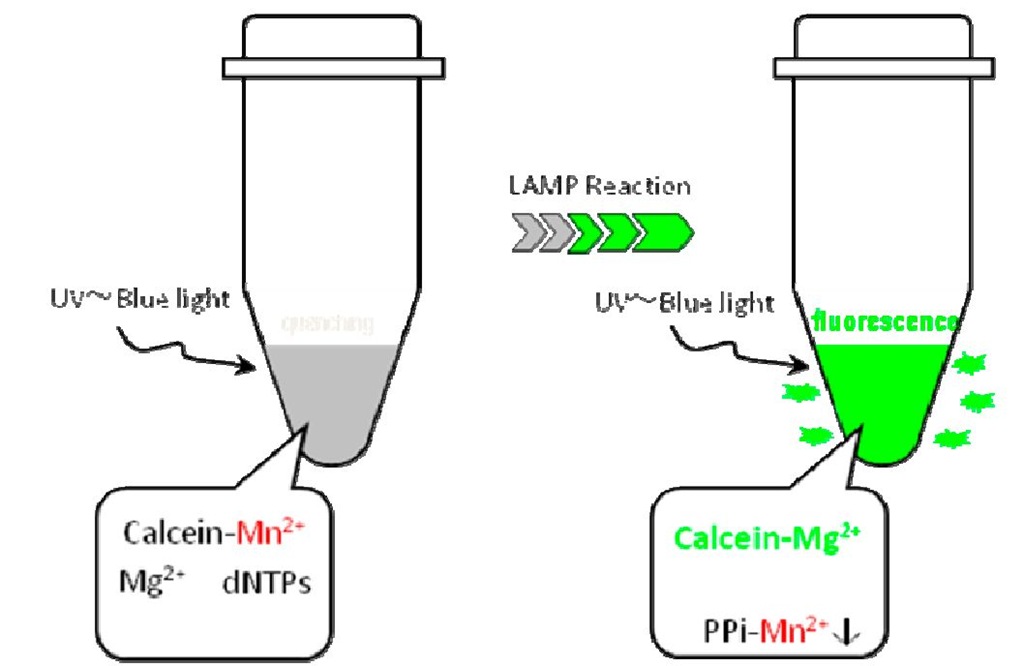
The results of the LAMP assay can be detected visually by observing the strength of the green fluorescence emitted after the reaction. Figure 2 represents the mechanism of the calcein method (Tomita et al., 2008). Before LAMP amplification, the metalochrome indicator "calcein" is quenched by the effect of a manganese ion. After the LAMP reaction, pyrophosphate ions (PPi) are produced as a by-product of polymerase reaction; PPi subsequently forms a manganese pyrophosphate complex, causing the removal of the manganese ion from calcein, because the PPi are a stronger base than calcein. Next, free calcein combines with a magnesium ion to produce bright fluorescence. This technology enables the detection of LAMP reactions without the use of fluorescence detectors, which are usually expensive and difficult to manage in resource-limited settings. Other technologies for visual detection using LAMP have also been reported (Tao et al., 2011; Goto et al., 2009; Mori et al., 2006).
Fig. 2. Mechanism involved using calcein 2.3 Sample preparation – PURE
The sample processing method is the next important step in molecular diagnostics. Silica-based methods are well known and have been applied to a wide variety of samples, including blood and tissue (Bendall, 2002). However, these methods are unsuitable for resource-limited facilities due to the cumbersome procedures involved, including washing with organic solvents using high-speed centrifugation. Therefore, we have developed a simple and swift sample processing method named PURE. Thus far, it has been confirmed that PURE can be successfully applied to sputum, blood, serum, and swab samples. The mechanism of PURE is described below:
1. An aliquot of sample (blood, sputum, etc.) is added to the alkaline-based extraction solution and treated by heat to lyse the pathogens.
2. The sample solution is treated with adsorbent powder to remove inhibitory materials contained in samples and to neutralize the solution without any loss of target DNA.
3. After separating the solution from the powder by filtration, the obtained filtrate containing target DNA molecules can be used for reconstituting dried LAMP reagents, which are deposited to the lids of LAMP reaction tubes.
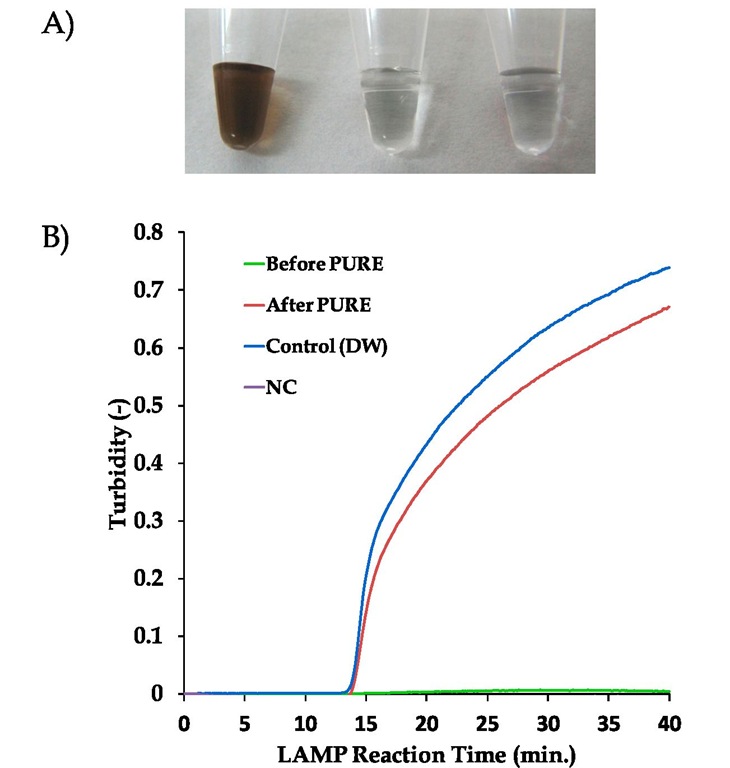
Fig. 3. Performance of the PURE-LAMP system applied for blood processing
A) Pictures of the blood sample solution (3.6% blood in an alkaline-based extraction solution) before and after PURE treatment. Left, Before PURE treatment; Centre, After PURE treatment; Right, distilled water (reference).
B) LAMP kinetics obtained by real-time turbidimetry for the 3 sample solutions with 1,000 copies of a template DNA spiked prior to PURE treatment.Green line, Before PURE treatment (directly added the solution to LAMP reaction); Red line, after PURE treatment; Blue line, control (distilled water); Purple line, negative control.
Figure 3 shows the performance of the PURE method applied for blood processing. An aliquot of blood was mixed with the extraction solution and heated at 70 °C for 5 minutes. Almost colorless solutions have been obtained by mixing the solution with the adsorbent powder (Fig. 3-A). The graph of real-time turbidimetry (Mori et al., 2004) in figure 3-B shows the LAMP kinetics for the 3 samples using 1,000 copies of a spiked template DNA. Untreated blood samples did not provide a positive reaction due to the inhibition from blood and extraction solution. However, PURE-treated blood samples showed almost the same kinetics as those of distilled water, indicating that PURE can remove inhibitory materials quite effectively from the blood samples without any loss of DNA.
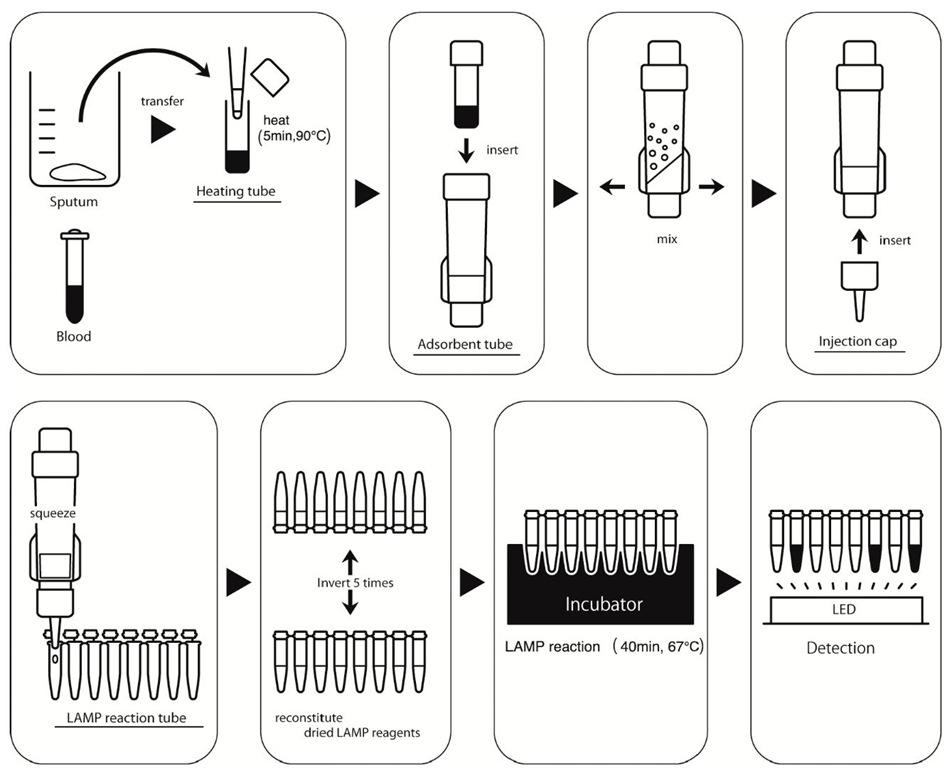
Figure 4 shows the overall process of the PURE-LAMP assay system. First, an aliquot of sample is placed in a heating tube and heated at an optimized temperature (70-90 °C) to lyse the target pathogen. Then, the heating tube is attached to an adsorbent tube, and the treated solution is vigorously mixed with adsorbent powder. Next, the injection cap is inserted into the absorbent tube, which is then squeezed to elute the solution containing purified DNA. These processes can be performed in approximately 10 minutes or less for a particular sample. The LAMP reagents deposited in the lid of the tube are reconstituted by shaking the tube several times and then incubating it at around 65 °C for 30-40 minutes. Finally, the results of amplification are detected by simply observing the fluorescence using the LED lights provided in the incubator. As mentioned above, we have successfully developed simple technologies for all the 3 steps required in molecular diagnostics, that is, the PURE method for sample preparation, the LAMP for amplification, and the calcein method for detection. Therefore, a combination of these technologies can be considered as a platform for a new molecular diagnostic tool with the desired simplicity.
Application of the newly developed platform for diagnosing tropical diseases
The developed platform has been applied to the following 3 tropical diseases to evaluate its performance as a practical diagnostic system.
Malaria and human African trypanosomiasis
Malaria is 1 of the 3 major infectious disease endemics in most tropical countries. More than 500 million people have been infected, and more than 1 million people die from malaria each year, mostly infants and pregnant women. Of the 4 malaria causing species, Plasmodium falciparum often causes severe, acute, and fatal malaria. In most developing countries, malaria is confirmed mainly by a blood smear test, although the sensitivity of the test is not sufficient to detect the parasites in patients with early-stage malaria. Dried LAMP reagents using P. falciparum (Pf)-specific primers and pan genus (Pg) primers were developed in this study. The Pg LAMP primers were designed on the basis of the homogeneous sequence shared by all the 4 malaria species, thus providing the same primer specificity for all the 4 species. The Pf-specific LAMP primers were designed based on mutations between the Pf sequence and the other 3 sequences, making the primer specific only to P. falciparum. If both Pf and Pg LAMP assays give positive results, it can be interpreted that the patient is infected by P. falciparum. On the other hand, if only the Pg LAMP assay gives positive results, the patient can be diagnosed with malaria caused by 1 or more of the other 3 malarial parasites.
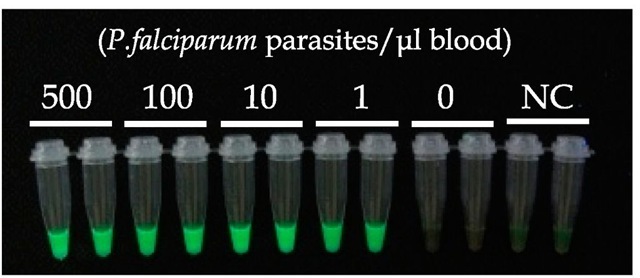
The sensitivity of PURE and malaria-LAMP have been evaluated by using of cultured P. falciparum parasites obtained from the American Type Culture Collection (ATCC). As shown in figure 5, both Pf and Pg malaria-LAMP assays can detect down to 1 parasite in 1 μ! blood, processed by the PURE method. This sensitivity of 1 parasite/μl blood is much higher than that of smear microscopy test (~50 parasites/μl blood for routine tests in an endemic area (Moody, 2002)). It has been reported that the initial malarial symptoms appear after the accumulation of approximately 1,000 parasites/ml of blood (Andrews et al., 2005). Therefore, PURE-malaria-LAMP is sensitive enough to detect parasites in patients who present with the initial symptoms of malaria.
Human African trypanosomiasis (HAT) is one of the most neglected disease endemics in central African countries (Hotez, 2007). HAT is caused by an infection of protozoa Trypanosoma brucei gambiense or Trypanosoma brucei rhodesiense transmitted by tse-tse flies. The symptoms of HAT occur in 2 phases: hemolymphatic phase, followed by the neurological phase. If left untreated, the hemolymphatic phase permits the parasites to invade the central nervous system of the patient, resulting in fatal neurological symptoms such as coma and eventually death. Because no vaccine or preventative drug for HAT is available, and therapeutic drugs for HAT patients in the neurological phase causes severe side effects, a simple and sensitive diagnostic method is in high demand.
Fig. 4. Diagram of the procedures involved in the PURE-LAMP system
Fig. 5. Sensitivity of PURE-Malaria-LAMP
Thirty-five microliters of control blood spiked with cultured parasites (P. falciparum, from ATCC) was treated with PURE and tested by P. falciparum-specific and Pan-genus LAMP. Left and right tubes contain the same parasite numbers and are those of Pf-LAMP and Pg-LAMP, respectively.
To enhance sensitivity, the LAMP primers for HAT are designed on a multi-copy gene named RIME, which contains 80-250 copies in a parasite genome (Njiru et al., 2008). The efficacy of the PURE-HAT-LAMP assay was also evaluated by using uninfected control blood spike with cultured parasites. Positive results have been successfully obtained from samples with a parasite density of 100/ml, indicating that the overall analytical sensitivity of PURE-HAT-LAMP can be estimated to be approximately 100 parasites/ml blood. The sensitivities of the currently available smear microscopy tests have been reported to be between 100 and 10,000 parasites/ml blood (Chappuis., 2005). The sensitivity of PURE-HAT-LAMP has been found to be over 10 times higher than that of the simple Giemsa-stained smear microscopy test, which is one of the most common diagnostic tests in HAT endemic areas, and is almost comparable with that of the mini-anion-exchange centrifugation technique, which is quite tedious and time consuming.
Tuberculosis
Tuberculosis (TB) is one of the most threatening airborne diseases worldwide. One-third of the world’s population is thought to be infected with Mycobacterium tuberculosis and new infections occur at a rate of about 1/second. Since the TB patients are concentrated in developing counties, including many tropical countries, TB is considered as a major poverty-related disease (Walker et al., 2003).
The sputum direct smear microscopy test is the only diagnostic method available for the detection of TB at peripheral laboratories in developing countries (Keeler et al., 2006). Because of the low sensitivity of the smear test, few patients with a low number of infected TB cells are often misdiagnosed as negative, thus preventing eradication of TB. In order to overcome this situation, we attempted to apply the novel platform PURE-LAMP system for the diagnosis of TB.
As summarized in Table 1, PURE-TB-LAMP provided positive results for both smear- and culture-positive sputum samples collected from patients suspected with TB. Furthermore, the PURE-TB-LAMP assay can detect the pathogen with 60% accuracy in smear-negative but culture-positive samples, and with 75% or more accuracy if 20 colonies are detected by the culture test using Ogawa media. This data clearly shows that the PURE-TB-LAMP method is reliable enough to be applied to the targeted peripheral smear centers in developing countries as an alternative method for the direct smear test.
|
Direct Smear |
Culture(Ogawa) |
PURE-TB-LAMP (Positive/Total) |
% positive |
|
Positive |
Positive |
34/34 |
100 |
|
Positive |
15/25 |
60 |
|
|
Negative |
100-200 |
7/7 |
100 |
|
20-99 |
6/8 |
75 |
|
|
1-19 |
2/10 |
20 |
|
|
Negative |
Negative |
9/91 |
9.9 |
Table 1. Summary of clinical performance of PURE-TB-LAMP
Sixty microliters of sputa obtained from patients suspected with TB in the Pham Ngoc Thach Hospital (PNTH; Vietnam) were analyzed by PURE-TB-LAMP. The performance of PURE-TB-LAMP was compared to those of the direct smear and culture method (Ogawa media) in terms of the positive ratios. Smear and culture tests for each sample were conducted according to the standardized protocols in PNTH.
Conclusions
As mentioned above, PURE and LAMP system have been newly developed and successfully applied as a novel diagnostic platform for the detection of infectious diseases, which are wildly endemic in the developing world. This platform has the advantage of being simple enough to be applicable in resource-limited facilities and its performances is higher than those of the existing diagnostic methods routinely employed in rural laboratories of most of the developing countries. Recently, a novel idea for performing LAMP without electricity has been proposed (LaBarre et al., 2011). Combination of that technology with the platform mentioned in this topic would make it possible to realize the use of molecular diagnostics in poorer settings or even in field conditions.
Since the geographical distribution of malaria, HAT, and TB overlap in many of tropical countries (Cook & Zumla, 2003), diagnostic tests for these diseases are often performed at the same rural laboratory in developing countries. The developed platform described in this study is a very useful tool in such laboratories because all the above mentioned diseases can be diagnosed using almost the same technique and the same simple incubator. This new technology can be beneficial as it reduces the initial costs associated with installing new equipments and preparing trained technicians for each target. This platform is potentially applicable to other pathogens, including those causing other neglected diseases such as leishmaniasis and Chagas’ disease. The application of this platform could be extended to other diseases that threaten the heath and quality of life of patients in many tropical countries. This can also contribute to distribute them at more affordable rates because of the effect of mass production.
This platform can be considered as a gene point-of-care testing (g-POCT) device, which can also be used in developed countries. In fact, TB-LAMP has been approved as clinical in vitro diagnostics (IVD) in Japan and used along with PURE as a simple and fast screening test for patients suspected with TB. Since NALC-NaOH treatment for sputum is not necessary for PURE-TB-LAMP, turn-around-time of PURE-TB-LAMP is less than that of the decontaminated smear test, which is commonly adopted as the standard screening test for TB in most developed countries. Furthermore, LAMP reagents using similar concepts have been developed for the detection of the influenza virus (Nakauchi, 2011). We hope that the platform will contribute to the improvement of global health and benefit all those under the threat of infectious diseases.