Introduction
Lassa fever is a frequently underestimated but socially and economically devastating disease. Lassa fever first came into limelight in 1969, when two nuns died as a result of complications of a hemorrhagic fever in Lassa town in the Borno State of Nigeria. Since then it has become endemic in many parts of West Africa. Out breaks of Lassa fever are usually associated with high mortality rates as the cases usually present late to the hospitals. Besides, many doctors find it difficult to diagnose Lassa fever until complications have set in because of the similarity of presentation to other more common febrile illnesses such as malaria and typhoid. In a study carried out in Sierra Leone in 1987, Lassa fever was found to be responsible for 10-16% of admissions and 30% of deaths in a major referral center. In another study of adult medical admissions in a special center for the management of Lassa fever in Nigeria in 2008, Lassa fever was responsible for 7% of admissions and 13% of deaths with a case fatality rate of 28%. However, the enlightenment campaign for the prevention of Lassa fever and diagnostic facilities are either lacking or rudimentary in most countries where Lassa fever is endemic. Compared to HIV/AIDS, Lassa fever is more infectious to close associates and it rapidly kills in dozens. However, Lassa fever does not get the global attention it deserves.
Etiology/pathogenesis
Lassa fever is caused by Lassa fever virus, a member of the family Arenaviridae. It is an enveloped single stranded bi-segmented rna virus Replication for Lassa virus is very rapid, while also demonstrating temporal control in replication. There are two genome segments. The first replication step is transcription of messenger RNA copies of the negative- or minus sense genome. This ensures an adequate supply of viral proteins for subsequent steps of replication, as proteins known as N and L are translated from the mRNA. The positive- or plus-sense genome then makes viral complementary (vcRNA) copies of itself, which are + sense. The vcRNA is a template for producing minus sense progeny but mRNA is also synthesized from it. The mRNA synthesized from vcRNA are translated to make the G (spike) proteins and Z proteins. Thus, with this temporal control, the spike proteins, which are on the outside of the virus particle, are produced last, making the infection more difficult for the host immune system to detect. Nucleotide studies of the genome have shown that Lassa has four lineages: three found in Nigeria and the fourth in Guinea, Liberia, and Sierra Leone. The Nigerian strains seem likely to have been ancestral to the others but further research is required to confirm this.
The Lassa virus gains entry into the host cell by means of the cell-surface receptor the alpha-dystroglycan (alpha-DG), a versatile receptor for proteins of the extracellular matrix. It shares this receptor with the prototypic arenavirus lymphocytic choriomeningitis virus. Receptor recognition depends on a specific sugar modification of alpha-dystroglycan by a group of glycosyltransferases known as the LARGE proteins. Specific variants of the genes encoding these proteins appear to be under positive selection in West Africa where Lassa is endemic. Alpha-dystroglycan is also used as a receptor by viruses of the New World clade C arenaviruses (Oliveros and Latino viruses). In contrast, the New World areanviruses of clades A and B, which include the important viruses Machupo, Guanarito, Junin, and Sabia in addition to the non pathogenic Amapari virus, use the transferrin receptor 1. A small aliphatic amino acid at the GP1 glycoprotein amino acid position 260 is required for high-affinity binding to alpha-DG. In addition, GP1 amino acid position 259 also appears to be important, since all arenaviruses showing high-affinity alpha-DG binding possess a bulky aromatic amino acid (tyrosine or phenylalanine) at this position.
Unlike most enveloped viruses which use clathrin coated pits for cellular entry and bind to their receptors in a pH dependent fashion, Lassa and lymphocytic choriomeningitis virus instead use an endocytotic pathway independent of clathrin, caveolin, dynamin and actin. Once within the cell the viruses are rapidly delivered to endosomes via vesicular trafficking albeit one that is largely independent of the small GTPases Rab5 and Rab7. On contact with the endosome pH-dependent membrane fusion occurs mediated by the envelope glycoprotein.
Lassa virus will infect almost every tissue in the human body. It starts with the mucosa, intestine, lungs and urinary system, and then progresses to the vascular system.
Predisposing factors
• Use of rat meat as a source of protein by people in some communities; contamination of exposed food by rat feces and urine;
• Traditional autopsy, where the operator may injure himself with scalpel and contaminate the injury with the blood of the deceased, who may have died of Lassa fever
• Forceful ingestion of water used in bathing a dead husband, by a widow suspected to be involved in his death. In many communities, family members may be forced to drink water used in bathing dead relatives in order to prove their innocence.
• Corrupt practices by staple food producers, which involve drying garri (cassava flour) in the open air in the daytime and sometimes at night. This enables all types of rat including mastomys natalensis to contaminate the flour with their excreta. This constitutes a public health hazard when the infected garri is sold to consumers in the market. The common habit of eating garri soaked in water may favor Lassa fever infection.
• Many other types of staple foods are also processed in the open sun, which is the major natural drier. These include rice, plantain chips, yam chips and cassava chips, which are processed into rice flour, plantain flour, yam flour, and raw cassava flour. Though these are also processed into staple foods such as tuwo shinkafa, plantain based amala, yam based amala and lafun respectively, the amount of heat involved in processing them into edible pastes, may be enough to denature lassa fever virus, which is heat labile.
• Bush burning of savannahs may be carried out by meat-hungry youths, during the dry season, in order to be able to have access to rodents and other animals. This habit often drives mastomys natalensis, the reservoir of lassa fever virus, into peoples homes and may be responsible for outbreaks of lassa fever in the dry season.
Epidemiology
Distribution
Lassa fever is endemic in West Africa. However the world is now a global village and the previous geographical gap between the tropics and the developed world has been bridged by international travel. The 6 – 21 days incubation period indicates that a person who contacts Lassa fever in an endemic area in West Africa may travel to a developed country within the incubation period and cause an epidemic.
Prevalence
The prevalence of Lassa fever can be assesed by determining the prevalence of antibodies to Lassa fever in communities. The prevalence of Lassa fever in Nigeria, Guinea and Sierra Leone can be up to 21%, 55% and 52% respectively.
Reservoir
The reservoir of infection is mastomys natalensis. It is a species of rodent in the Muridae family. It is also known as the Natal multimammate rat, the common African rat, or the African soft-furred rat. It is found in Angola, Benin, Botswana, Burkina Faso, Burundi, Cameroon, Central African Republic, Chad, Republic of the Congo, Democratic Republic of the Congo, Ivory Coast, Equatorial Guinea, Ethiopia, Gabon, Ghana, Guinea, Guinea-Bissau, Kenya, Lesotho, Malawi, Mali, Mauritania, Mozambique, Namibia, Niger, Nigeria, Rwanda, Senegal, Sierra Leone, Somalia, South Africa, Sudan, Swaziland, Tanzania, Togo, Uganda, Zambia, and Zimbabwe. Its natural habitats are subtropical or tropical dry forests, subtropical or tropical moist lowland forests, dry savanna, moist savanna, subtropical or tropical dry shrubland, subtropical or tropical moist shrubland, arable land, pastureland, rural gardens, urban areas, irrigated land, and seasonally flooded agricultural land. In 1972, the Natal multimammate Mouse was found to be the natural host of the deadly Lassa fever virus.
Transmission
Lassa fever is transmitted to humans when they ingest food contaminated by the feces and urine of mastomys natalensis. Once humans are infected, transmission also occurs from human to human through contact with fluid and aerosol secretions in the form of sneezing, sputum, seminal fluid, stool, urine and blood. Vertical transmission through breast milk has been observed.
Host factors
Men are more commonly affected than women. However the case fatality rate is nearly two times higher in women. Men are more likely to buy food from food vendors especially at lunch time while women are more likely to eat personally cooked food. Contamination of food from this source may be responsible for the higher incidence of Lassa fever in men. Although the high case-fatality of Lassa fever is due to delayed cellular immunity, development of partial immunity as a result of frequent exposure to contaminated food may be responsible for the milder forms of the disease and lower case-fatality rate in men. Research is needed to find out whether Lassa fever infection confers partial or full immunity on affected people.
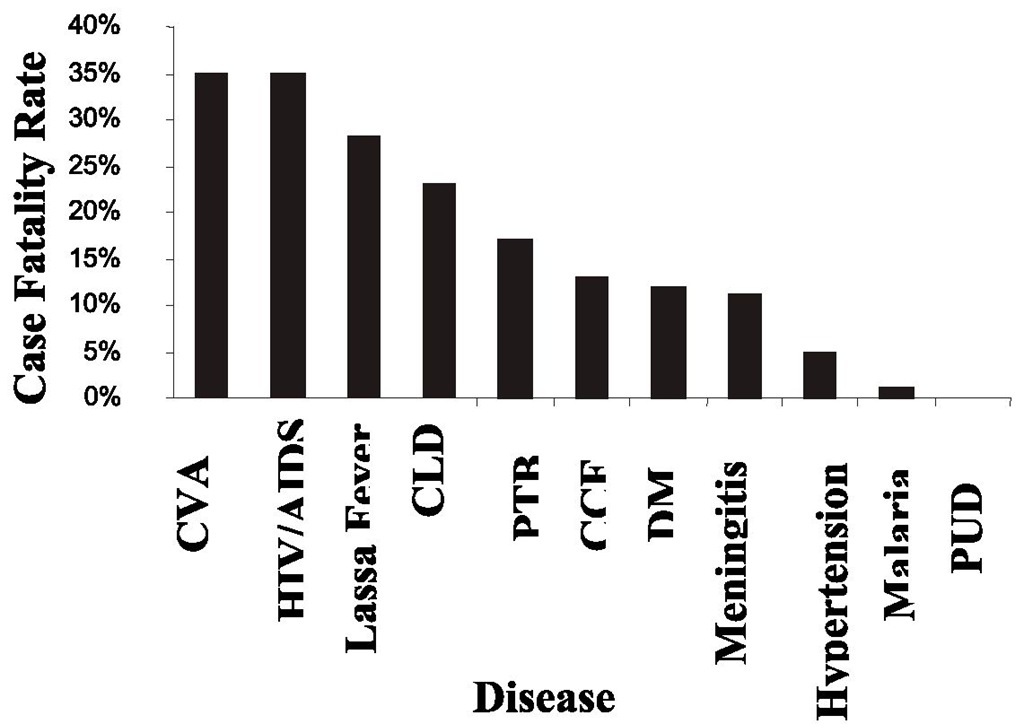
Fig. 1. Bar Chart showing Case Fatality Rates of Common Diseases of Medical Inpatients in Irrua Specialist Teaching Hospital (ISTH), Irrua, Nigeria in 2007
Clinical features
Signs and symptoms typically occur after an incubation period of 6 -21 days. The onset of illness is insidious, with fever and shivering accompanied by malaise, headache and generalized aching. Sore throat is a common early symptom. In some cases the tonsils and pharynx may be inflamed with patches of white or yellowish exudate and occasionally small vesicles or shallow ulcers. (Importantly, a similar appearance may be seen in cases of malignant tertian malaria). As the illness progresses the body temperature may rise to 41°C with daily fluctuations of 2-3°C. The duration and severity of fever is very variable. The average duration is 16 days but extremes of 6-30 days have been reported. A feature of severe attacks is lethargy or prostration disproportionate to the fever. During the second week of illness there may be edema of the head and neck, encephalopathy, pleural effusion and ascites. Vomiting and diarrhea may aggravate the effects of renal and circulatory failure. Severe cases develop significant hemorrhage and multi-organ failure with widespread edema and bleeding into the skin, mucosae and deeper tissues. In non-fatal cases, the fever subsides and the patient’s condition improves rapidly although tiredness may persist for several weeks. There is usually a leucopenia early in the course, though a high polymorphonuclear leucocytosis may occur with severe tissue damage. Another common late complication is sensorineural deafness. Clinically, a Lassa fever infection is difficult to distinguish from other viral hemorrhagic fevers, such as Ebola and Marburg, and from more common febrile illnesses such as malaria and typhoid.
Complications of Lassa fever
Various complications may occur in the course of Lassa fever. These complications vary with duration of illness and sex of the victim. Thes complications include hypovolemic shock, Electrolyte imbalance, Disseminated intravascular coagulation, Renal failure, Sensorineural deafness, pregnancy complications.
Hypovolemic shock
Lassa fever viremia causes endothelial and platelet dysfunction with consequent leaky capillary syndrome. Bleeding occurs in all organs and from all mucosae leading to hypovolemic shock.
Electrolyte imbalance
Most Lassa fever victims lose fluid through vomiting and diarrhea and therefore develop electrolyte imbalance.
Renal failure
Renal tubular damage may also occur on Lassa fever and in conjunction with the hypovolemic shock predispose to renal failure
Complications of lassa fever in pregnancy
Lassa fever is especially dangerous in pregnant women. Abortion is common in early pregnancy and intrauterine fetal death is common in later pregnancies. Abortion reduces the mortality rate in affected pregnant women. Prognosis is very poor in pregnant women as mortality rate may be up to 80%.
Sensorineural deafness
This is the commonest complication of Lassa fever. It is not related to the severity of disease as it may occur with the same frequency in both mild and severe forms of the disease.
Laboratory diagnosis
Lassa fever is most often diagnosed using ELISAs. The virus can also be detected by reverse transcription PCR (RT-PCR) in all patients by the third day of illness, but immunofluorescence identifies only 52% of the patients.
Treatment
Ribavirin, an antiviral drug, is the current treatment of Lassa fever. The drug is to be administered in a volume of 50-100 ml of normal saline to be infused over 30-40 minutes.
• Loading dose: 33 mg/kg (maximum dose 2.64 g)
• Followed by a dose of 16 mg/kg (max dose 1.28 g) every 6 hours for the first 4 days
• Followed by a dose of 8 mg/kg (maximum dose 0.64 g) every 8 hours for the subsequent 6 days
Supportive treatment is usually carried out with intravenous fluids, and treatment of complications such as renal failure and infections may be necessary.
Although Lassa fever can be treated with ribavirin, early diagnosis and treatment is essential in all cases of Lassa fever. Ribavirin is most effective when given within 6 days of illness. Self-diagnosis and treatment is common in the tropics because of ignorance and poverty. It is only when there is no remission of fever that the patient seeks treatment in a health-care facility. However, many health-care providers are unable to make early diagnosis, and are very likely to make a diagnosis of resistant malaria or typhoid. Besides most health care providers have no access to diagnostic facilities, which are only available in tertiary health centers. This allows the patient to get to terminal stages before they are transferred to a tertiary center. Sometimes the life of the health-care provider is claimed along with that of the patient.
Table 1. Case fatality rates of common Diseases among Medical inpatients in ISTH, Irrua, Nigeria.
Prognosis
Prognosis depends on how early a patient presents at the clinic. Most patients recover completely if diagnosed early and when treatment with ribavirin is commenced within 6 days of illness. In studies carried out in special referral centers in Nigeria and Sierra Leone, Lassa fever was responsible for 13% and 30% of adult deaths respectively. The death rates were in adult medical wards where only 7% and 10-16 % respectively of the total number of admissions were for Lassa fever. Prognosis is probably better in males who may acquire partial immunity due to the habit of patronizing food vendors. In a study done in Nigeria, the case fatality rate in males was 23% compared to women with 44%, though males were four times more commonly affected than females.
Control
The individual
The affected person should be admitted to a special center for the treatment of Lassa fever. Where this is not possible, the patient should be barrier-nursed. Health care providers and close associates of the patient should wear protective clothing, masks and gloves. Excrements from affected persons should be properly disposed.
The community
Legislation is needed to prevent widowhood rites, traditional autopsies, bush burning and unhygienic preparation of garri and other staple foods. Animal husbandry and fisheries should be encouraged in order to provide alternative sources of first-class proteins for rat eaters. Regular and sustainable environmental sanitation is needed to prevent rat breeding. The public should be made aware of the mode of contact of Lassa fever and its high case-fatality rate using print and electronic media. Community involvement and participation is necessary to provide sustainable Lassa fever control. Food vendors should be educated on the need to prevent food contamination with Lassa fever virus. Grains, flours and left-over foods should be adequately covered to prevent contamination by rats. Rodenticides should be used for the destruction of rats in homes, and development of Lassa fever vaccine should be facilitated. Regular seminars should be held for health-care providers on early diagnosis and treatment of Lassa fever, while diagnostic kits should be made available in district hospitals. Affected people should be referred early to the special center in order to prevent or limit disability, while those with disabilities should be rehabilitated functionally, socially and psychologically so that they can be gainfully employed.
Prospects
Though vaccines are not currently available for Lassa fever, there is evidence that they will be produced in the near future. Research done with non-human primates have revealed that survivors exhibit fewer lesions and a lower viral load than non-survivors.
Although all animals develop strong humoral responses, antibodies appear more rapidly in survivors and are directed against GP(1), GP(2), and NP. Activated T lymphocytes circulate in survivors, whereas T-cell activation is low and delayed in fatalities
A single injection of ML29 reassortant vaccine for Lassa fever induces low, transient viremia, and low or moderate levels of ML29 replication in tissues of common marmosets depending on the dose of the vaccination. The vaccination elicits specific immune responses and completely protects marmosets against fatal disease by induction of sterilizing cellmediated immunity. DNA array analysis of human peripheral blood mononuclear cells from healthy donors exposed to ML29 revealed that gene expression patterns in ML29-exposed PBMC and control, media-exposed PBMC, clustered together confirming safety profile of the ML29 in non-human primates. The ML29 reassortant is a promising vaccine candidate for Lassa fever.