Introduction
The transmission of the parasites that cause human malaria is influenced by myriad environmental factors, including changes in agricultural practices, deforestation, and water-resources development and management (Ijumba and Lindsay, 2001; Ijumba et al., 2002; Keiser et al., 2005; Guerra et al., 2006), climatic factors such as rainfall, humidity and temperature (Reiter, 2008), and various cultural, economic, political and social factors, including health-seeking behaviour, urbanization, armed conflict and war (Esse et al., 2008; Baragatti et al., 2009). Drug resistance in the causative parasites and insecticide resistance in the mosquito vectors are also important factors that now influence malaria transmission (Reiter, 2008). Each year, in many parts of Africa, the local populations of anopheline mosquitoes build up rapidly and peak shortly after the onset of the rainy season (Mbogo et al., 1995). In two studies on the relationships between mosquito abundance, malaria transmission and rainfall in West Africa, 70%-90% of the children investigated were found infected with Plasmodium spp. after the rainy season (Bonnet et al., 2002; Koudou et al., 2009). It is particularly during and at the end of the rainy season that malaria becomes one of the leading causes of mortality and health-seeking at dispensaries and hospitals in this region (Rey et al., 1987). Not only season but also changing patterns of agriculture, particularly irrigated rice farming, influence malaria transmission in Africa (Ijumba & Lindsay, 2001; Diuk-Wasser et al., 2007; Sogoba et al., 2007).
Additionally, malaria transmission, Plasmodium prevalence rates, the proportion of presumptive and clinically-confirmed malaria episodes have been studied in two villages of central Côte d’Ivoire: one with irrigated rice farming (Zatta) and one without (Tiémélékro) (Koudou et al., 2009). Due to a farmers’ conflict over land and socio-political issues, irrigated rice farming was interrupted in Zatta in 2003.
Methods
Study sites
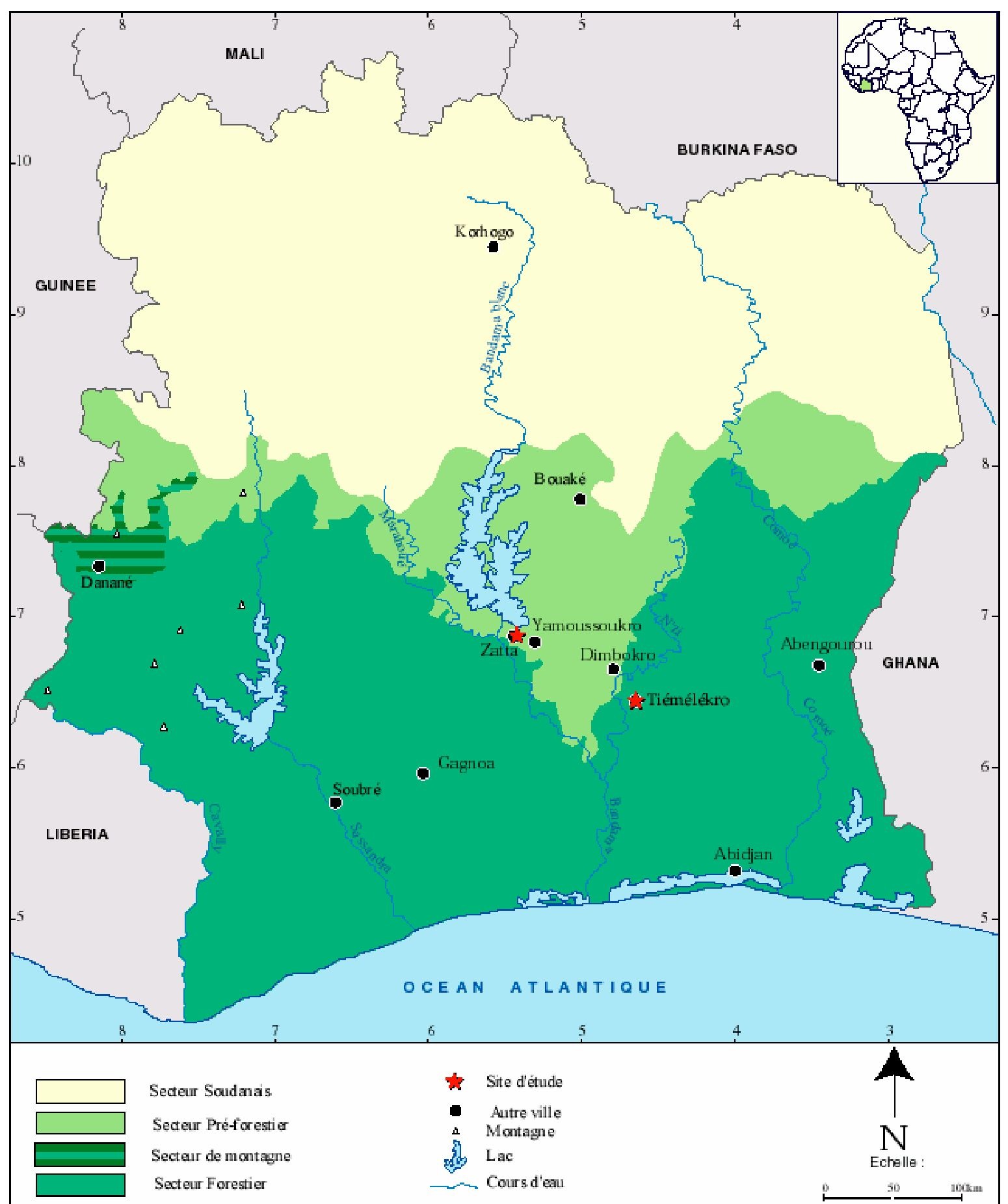
The study described here was carried out in the villages of Tiemelekro (geographical coordinates: 6°500 N, -4°170 W) and Zatta (6°880 N, -5°390 W), located in central Côte d’Ivoire (Figure 1). A detailed description of Tiemelekro, including climatic conditions, current health care delivery structures and key demographic and socioeconomic indicators, has been presented recently (Girardin et al., 2004). Zatta is located 7 km north-west of Yamoussoukro, the capital city of Côte d’Ivoire. The mean annual temperature in this village is 26.5°C and the mean annual precipitation is 1280 mm. There is a long rainy season between April and July and a shorter one in October/November. A dispensary, run by two local nurses, is located in Zatta and also covers nearby settlements. Two small dams were constructed in this village in the mid-1970s. Since 1997, a very large irrigated rice field has been cultivated on an estimated surface area of 36 ha, in close proximity to human habitations. However, due to unstable socio-political conditions and a farmers’ conflict over land, rice irrigation was interrupted in 2000 and again in 2003/2004.
Living conditions and several of the investigated household characteristics are comparable between the two study villages. For example, similar proportions of houses utilized iron-corrugated sheets as roofing material (93.8% in Tiemelekro vs. 92.9% in Zatta), and had running water at home (74.1% vs. 65.4%). On the other hand, improved sanitation facilities were less prominent in Tiemelekro than in Zatta (17.0% vs. 47.6%). With regard to personal protective measures against mosquito bites, the proportion of people sleeping under a bednet was similarly low in both villages (8.4-11.2%), whereas use of fumigating coils was much more pronounced in Zatta (47.3%) when compared to Tiemelekro (9.1%).
Rainfall data collection
The Ivorian "Societe d’Exploitation et de Developpement Aeroportuaire et Meteorologique" (SODEXAM) holds rainfall data for the study area, from 1971 onwards. For the present study, monthly rainfall data from 2002 to 2005 were extracted from the society’s records.
Adult mosquitos’ collection
Overall, 13 entomological surveys were carried out: seven in the long rainy seasons (in the April and June of 2002, the April, May, June and July of 2003, and the May of 2005), and six in dry seasons (in the February and August of 2002, 2003 and 2005). Adult mosquitoes were collected by means of human-bait night catches. The surveys in 2002 and 2005 were each conducted between 18.00 and 06.00 hours, both inside and outside sentinel houses. Each of the six surveys carried out in 2003, however, covered shorter time periods (from 22:00 to 06:00 hours) and the collectors were only stationed inside the sentinel house because of the unstable socio-political situation at the time. Overall, 96, 48 and 32 night catches were carried out in 2002, 2003 and 2005, respectively. No surveys could be undertaken in 2004, as it was then considered too dangerous to reach the study villages.
Laboratory procedures
Adult mosquitoes were brought to a laboratory and processed. Firstly, the physiological age of each female Anopheles and the corresponding parity rate (i.e. the proportion of female mosquitoes that had laid eggs at least once) were determined by dissection of ovaries and examination of tracheoles (Detinova, 1962). For quality control, a random sample of 10% of the mosquitoes investigated were re-examined by a senior technician. Secondly, a proportion of An. gambiae female having laid eggs at least once was checked for P. falciparum infection, in an ELISA for detecting the parasite’s circumsporozoite protein (Beier et al., 1988).
Fig. 1. Vegetation mapping of Côte-d’Ivoire presenting both study sites (Zatta and Tiemelekro) located in the central part of the country.
Thirdly, some of the females belonging to the An. gambiae complex were identified to species level, in a PCR-based assay (Scott et al., 1993). Finally, some of the mosquitoes belonging to the An. funestus group were further identified using an assay based on a multiplex PCR (Koekemoer et al., 2002; Cohuet et al., 2003).
Clinical and Parasitological surveys
Repeated cross-sectional surveys were carried out in the study villages to assess malaria parasitaemia and clinical malaria in children aged <15 years. The first survey was done in June 2002. In 2003, two surveys were carried out in Zatta and three in Tiémélékro. The research team first worked in the primary schools and all children aged between 7 and 15 years from randomly selected classes were invited for a finger prick blood sample. Next, mothers and caregivers of under 7-year-old children were invited to accompany their children to a designated community location where a blood sample was taken from each child.
Thick and thin blood films were prepared on microscope slides. The slides were air-dried prior to transfer to a nearby laboratory where they were stained with Giemsa for 45 min. The slides were examined by the same experienced laboratory technician throughout the study under a microscope at high magnification. Plasmodium species and gametocytes were identified and counted against 200 leucocytes. When less than 10 parasites were found, reading was continued for a total of 500 leucocytes. Parasitaemia was expressed by the number of parasites per μ! of blood, assuming for a standard count of 8000 leucocytes/ μ! blood. For quality control, 10% of the slides were randomly selected and re-examined by a second senior technician.
In our study, fever was defined when an individual had an axillary temperature >37.5 °C. Clinical malariawas defined as fever plus parasitaemia (Smith et al., 1994). Particular emphasis was placed on clinical cases with a parasitaemia >5000 parasites/_l blood. The latter threshold has been chosen after comparing the proportions of fever cases and asymptomatic carriers for different classes of parasite density (Gaye et al., 1989). Subjects with malaria-related symptoms (e.g. headache) plus axillary temperature >37.5 °C were given artesunate plus amodiaquine (the respective first-line antimalarial treatment at the time of the study) and paracetamole.
Ethical issues
The study protocol was approved by the institutional research commission of the Centre Suisse de Recherches Scientifiques (Abidjan, Côte d’Ivoire). Ethical clearance was obtained from the Ivorian Ministry of Public Health and National Malaria Control Programme. People who acted as bait and collectors in the mosquito collections were all volunteers and signed informed consent forms. During the study, Patients with malaria-related symptoms who presented at the dispensaries and mosquitoes’ collectors were treated and protected for free against malaria by artesunate-amodiaquine chemoprophylaxis (artesunate-amodiaquine being the recommended, first-line treatment for malaria in Côte d’Ivoire at the time of the present study) and all mosquitoes’ collectors were immunized against yellow fever. The heads of household in both study sites were informed and the parents or legal guardians of participating children signed a written informed consent sheet.
Results
Species composition of An. gambiae complex and An. funestus group
A total of 110 mosquitoes were identified to species level by PCR: 60 from Tiémélékro and 50 from Zatta. Within Anopheles spp. morphologically identified as An. gambiae complex, 100% were An. gambiae s. s. With regard to the An. funestus group, it consisted of 100% An. funestus s. s.
Effects of agricultural practices (irrigated rice fields & vegetable farming) on Plasmodium transmission
Comparison between years revealed that the biting rate of An. gambiae s.l. in Zatta decreased several-fold from 49.3 bites per person per night (b/p/n) in 2002 to 7.9 b/p/n in 2003 (likelihood ratio test (LRT=1072.66; P<0.001). In Tiemelekro, the biting rates recorded in 2002 and 2003 remained fairly constant. These observations were paralleled by a marked decrease in the infective rate of An. gambiae s.l. in Zatta (4.6-1.2%), and an increase in Tiemelekro (3.17.6%). Meanwhile, the entomological inoculation rate (EIR) of An. gambiae s.l. decreased 21fold in Zatta, from 789 to 38 infective bites per person per year (ib/p/y), whereas it remained high in Tiemelekro (233 vs. 342 ib/p/y). In Zatta, the return to irrigated rice farming in January 2005 was paralleled by a significant increase of the EIR ranging from 38 infective bites per person per year (ib/p/y) in 2003 to 295 ib/p/y in 2005. In Tiémélékro high EIRs were found in 2003 (342 ib/p/y) and 2005 (572 ib/p/y).
Effects of agricultural practices (irrigated rice fields & vegetable farming) on Plasmodium prevalence and clinical malaria cases
Irrigated rice fields and Plasmodium prevalence
In both villages, the peak prevalence of P. falciparum was generally observed in children aged 3-6 years. There were three exceptions: in Tiémélékro, the peak prevalence of P. falciparum during the May 2005 survey was found in the youngest age group (< 2 years), whereas in Zatta, the highest prevalence in the baseline survey (June 2002) and the second last survey (May 2005) was observed in children aged 7-15 years.
In June 2002, similarly high P. falciparum prevalence rates were observed in Zatta (85.4%) and Tiémélékro (86.1%). In Zatta, a significant decrease in the mean P. falciparum prevalence rate occurred from 2002 to 2003 (58.4%; χ2 = 42.33, degree of freedom (df) = 1; P < 0.001). There was a significant increase from 2003 to 2005 (66.0%; χ2 = 14.78, df = 1, P = 0.012). In Tiémélékro, the P. falciparum prevalence rate in June 2003 (78.2%) was significantly lower than during the June 2002 survey (χ2 = 4.92, df = 1; P = 0.027). The annual P. falciparum prevalence rate decreased significantly from 2003 (70.7%) to 2005 (60.4%; χ2 = 17.27, df = 1; P < 0.001).
Fever cases and asymptomatic carriers, stratified by parasite density
Table 1 shows how many of the children examined with parasitaemia in the 2003 surveys were either asymptomatic carriers or presented with a fever. There was a strong seasonal variation in the proportion of fever cases among individuals with parasitaemia. In Zatta, for example, the proportion of fever cases among Plasmodium-positive individuals was significantly higher towards the end of the rainy season (August) when compared to the dry season (March) (22.1% versus 9.9%; χ2 = 9.90, df = 1; P = 0.002). In Tiémélékro, considerably higher frequencies of fever cases among Plasmodium-positive individuals were recorded during the peak rainy season in June (27.3%) and towards the end of the rainy season in August (25.5%) when compared to the dry season in March (15.9%; P < 0.05 for both comparisons). In Zatta, all individuals with a high level of parasitaemia (> 5000 parasites/μl blood) presented with fever, accounting for a highly significant difference between the proportion of asymptomatic carriers and fever cases in this parasitaemia class (P < 0.001). Similarly, there was a highly significant association between the fever cases and high parasitaemia in the three surveys carried out in 2003 in Tiémélékro (P < 0.05). No statistically significant difference was found in children with lower parasitaemias (1000-5000 parasites^l of blood), neither in Zatta (March: χ2 = 1.53; df = 1; P = 0.068 and August: χ2 = 0.116; df = 1; P = 0.733) nor in Tiémélékro (March: χ2 = 0.18; df = 1; P = 0.671, June: χ2 = 2.23; df = 1; P = 0.135 and August: χ2 = 0.001; df = 1; P = 0.973).
Annual variation of presumptive cases and malaria transmission
In Zatta, 966, 812, 693 and 884 presumptive cases were recorded in 2002, 2003, 2004 and 2005, respectively. The annual number of presumptive malaria cases decreased significantly by 15.1% and 14.7%, respectively, from 2002 to 2003 (IRR = 0.841, P < 0.001) and from 2003 to 2004 (IRR = 0.853, P = 0.002). An opposite trend was observed from 2004 to 2005; the number of presumptive malaria cases increased significantly by 27.5% (IRR = 1.276, P < 0.001). The monthly number of presumptive cases was not related to the monthly number of infective bites per person (IRR = 0.994, P = 0.827).
|
Date of survey |
P. falciparum parasitaemia (parasites/μl blood) |
Tiémélékro |
|
Zatta |
|
|
No. (%) of asymptomatic carriers |
No. (%) of children with fever |
No. (%) of asymptomatic carriers |
No. (%) of children with fever |
||
|
March 2003 |
< 1000 |
69 (54.8%) |
13 (10.3%) |
135 (73.4%) |
9 (4.2%) |
|
1000-5000 |
37 (29.4%) |
6 (4.8%) |
57 (26.8%) |
9 (4.2%) |
|
|
> 5000 |
0 (0) |
1 (0.8%) |
0 (0) |
3 (1.4%) |
|
|
Total |
106 (84.1%) |
20 (15.9%) |
192 (90.1%) |
21 (9.9%) |
|
|
June 2003a |
< 1000 |
86 (52.1%) |
34 (20.6%) |
n.a. |
n.a. |
|
1000-5000 |
32 (19.4%) |
7 (4.2%) |
n.a. |
n.a. |
|
|
> 5000 |
2 (1.2%) |
4 (2.4%) |
n.a. |
n.a. |
|
|
Total |
120 (72.7%) |
45 (27.3%) |
n.a. |
n.a. |
|
|
August 2003 |
< 1000 |
99 (50.5%) |
22 (11.2%) |
78 (57.4%) |
12 (8.8%) |
|
1000-5000 |
46 (23.5%) |
16 (8.2%) |
28 (20.6%) |
7 (5.1%) |
|
|
> 5000 |
1 (0.5%) |
12 (6.1%) |
0 (0) |
11 (8.1%) |
|
|
Total |
146 (74.5%) |
50 (25.5%) |
106 (77.9%) |
30 (22.1%) |
|
|
Overall 2003 (number of positive children/ number of total children) |
54.0% (372/689) |
16.7% (115/689) |
49.8% (298/598) |
8.5% (51/598) |
|
Table 1. Number (%) of children infected with P. falciparum who were asymptomatic carriers or presented with fever, stratified by different levels of parasitaemia, in the two study villages of Tiémélékro and Zatta, central Côte d’Ivoire.
n.a.: not assessed
a No survey carried out in June 2003 in Zatta due to unstable sociopolitical situation
In Tiémélékro, the yearly numbers of presumptive malaria cases were 2089, 1858, 1655 and 1541. Thus, we observed significant decreases in the yearly number of presumptive cases by 11.1% from 2002 to 2003 (IRR = 0.889, P < 0.001), 9.0% from 2003 to 2004 (IRR = 0.910, P = 0.005) and 8.9% from 2004 to 2005 (IRR = 0.911, P = 0.008).
As in the case of Zatta, the monthly number of presumptive cases was not related to the monthly number of infective bites per person (IRR = 1.007; P = 0.776).