Today scientists know that these atoms are not, as the Greeks imagined, the smallest chunks of matter. Scientists quickly realized that atoms had multiple parts inside of them:
Negatively charged electrons circling the nucleus Positively charged nucleus
The particles that compose the nucleus (it’s made up of smaller pieces, too) and electrons are among the particles, along with several others, that the Standard Model of particle physics explains, and ultimately that string theory should also explain.
Discovering the electron
The electron is a negatively charged particle contained within the atom. It was discovered in 1897 by British physicist J.J. Thomson, though charged particles (including the name “electron”) had been hypothesized earlier.
Some physicists had already hypothesized that units of charge might be flowing around in electrical apparatus. (Benjamin Franklin proposed such an idea as early as the 1700s.) Technology only caught up to this idea in the late 1800s, with the creation of the cathode ray tube, shown in Figure 8-1.
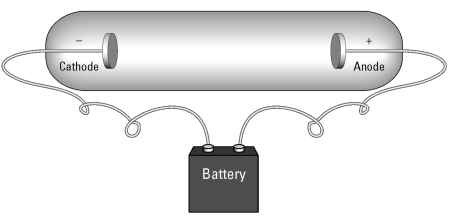
Figure 8-1:
Cathode ray tubes allow charged particles to be studied in a vacuum.
In a cathode ray tube, a pair of metal disks is connected to a battery. The metal disks are placed inside a sealed glass tube that contains no air — a vacuum tube. The electrical voltage causes one of the metal plates to become positively charged (an anode) and one to become negatively charged (the cathode, from which the device gets its name). Cathode ray tubes are the basis of traditional television and computer monitor tubes.
When the electrical current was switched on, the tube would begin to glow green. In 1897, Thomson was head of the Cavendish laboratory in Cambridge, England, and set about to test the properties of this cathode ray tube glow. He discovered that the glow was due to a beam of negatively charged particles flying between the plates. These negatively charged particles later came to be called electrons. Thomson also figured out that the electrons were incredibly light — 2,000 times lighter than a hydrogen atom.
Thomson not only discovered the electron, but he theorized that the electron was part of the atom (atoms weren’t a completely accepted idea at the time) that somehow got knocked free from the cathode and flowed through the vacuum to the anode. With this discovery, scientists began discovering ways to explore the inside of atoms.

The nucleus is the thing in the middle
In the center of the atom is a dense ball of matter, called a nucleus, with a positive electrical charge. Shortly after electrons were discovered, it became clear that if you extracted an electron from an atom, the atom was left with a slightly positive electrical charge. For a while, the assumption was that the atom was a positively charged mass that contained negative electrons inside of it, like pieces of negatively charged fruit in a positively charged fruitcake. The entire fruitcake would be neutral unless you extracted some fruit from it. (Scientists of the day, being of a different dietary constitution than most of us today, explained it as plum pudding instead of fruitcake. Plum pudding or fruitcake — it unappetizingly amounts to roughly the same picture.)
In 1909, however, an experiment by Hans Geiger and Ernest Marsden, working under Ernest Rutherford, challenged this picture. These scientists fired positively charged particles at a thin sheet of gold foil. Most of the particles passed straight through the foil, but every once in a while one of them bounced back sharply. Rutherford concluded that the positive charge of the gold atom wasn’t spread throughout the atom in the fruitcake model, but was concentrated in a small positively charged nucleus, and that the rest of the atom was empty space. The particles that bounced were the ones that hit this nucleus.
Watching the dance inside an atom
In trying to figure out the atom’s structure, a natural model for scientists to look to was the planetary model, as shown in Figure 8-2. The electrons move around the nucleus in orbits. Physicist Niels Bohr determined that these orbits were governed by the same quantum rules that Max Planck had originally applied in 1900 — that energy had to be transferred in discrete packets.
In astronomy, the Earth and sun are attracted to each other by gravity, but because Earth is in motion around the sun, they never come into contact. A similar model could explain why the negative and positive portions of the atom never came into contact.
The first planetary model was proposed in 1904 by Nobel Prize-winner Hantaro Nagaoka. It was based on the rings of Saturn and called the Saturnian model. Certain details of the model were disproved by experiment, and Nagaoka abandoned the model in 1908, but Ernest Rutherford revised the concept to create his own planetary model in 1911, which was more consistent with experimental evidence.
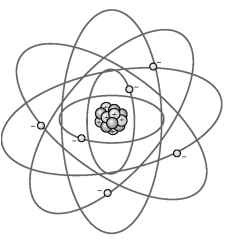
Figure 8-2:
The Rutherford-Bohr model of the atom has electrons moving in orbits around a positively charged nucleus.
When atoms emitted electrons, the electron’s energy followed certain precise patterns. Bohr realized in 1913 that this meant Rutherford’s model required some revision. To fit the patterns, he applied the idea that energy was quantized, or bundled together in certain quantities, which allowed for stable orbits (instead of the collapsing orbits predicted by electromagnetism). Each electron could only exist in a certain, precisely defined energy state within its orbit. To go from one orbit to a different orbit required the electron to have enough energy to jump from one energy state to another.
Because of the quantum nature of the system, adding half the amount of energy to go from one orbit to another didn’t move the electron halfway between those orbits. The electron remained in the first orbit until it received enough energy to kick it all the way into the higher-energy state. This is yet more of the strange behavior you’ve (hopefully) come to expect from quantum physics.
The Rutherford-Bohr model works pretty well in describing the hydrogen atom, but as atoms get more complex, the model begins to break down. Still, the basic principles hold for all atoms:

A nucleus is at the center of an atom. Electrons move in orbits around the nucleus.
The electron orbits are quantized (they have discrete energy levels) and are governed by the rules of quantum physics (though it would take several years for those rules to become developed, as described in
topic 7).
