Introduction
Spinal cord stimulation (SCS) is probably the most commonly performed surgical procedure for pain treatment in the United States, although the exact mechanism of its action remains largely unknown [1]. Several theories have been proposed to explain the pain suppressive effect of SCS. The most commonly accepted of these is the well-known "gate-control” theory of Melzack and Wall [2], which postulates the existence of a "gate” mechanism in the central nervous system that controls the processing and transmission of sensory information, including the nociceptive input. According to this theory, the impulse transmission in the nociceptive afferent pathway is modulated by activity in large-caliber myelinated non-nociceptive A-fiber affer-ents. Therefore, electrical stimulation of these large fibers anywhere along their course in the peripheral nerves or in the dorsal columns of the spinal cord can block central pain signaling.
An alternative theory explains the suppression of pain by a frequency-related conduction block that takes place at the branching point of the primary myelinated afferents into dorsal horns and dorsal column fibers [3]. In addition, clinical and experimental studies indicate that SCS may inhibit ischemic pain by improving regional blood flow [4] or decreasing tissue oxygen demands [5].
Whatever the underlying principle of SCS, the stimulation of the dorsal columns of the spinal cord produces pain relief in certain subsets of patients, and its success seems to correlate well with the production of stimulation-related paresthesias in the painful region [6].
Indications
The generally acceptable indication for SCS is neuropathic pain caused by injury to the nervous system either at or distal to the spinal cord (Table 1). This includes pain originating from peripheral nerve injury, neuropathies, postamputation stump pain, or complex regional pain syndromes (CRPS) types 1 and 2 (previously known as reflex sympathetic dystrophy and cau-salgia, respectively). Pain originating from the nerve roots, as in arachnoiditis or radiculopathies, also responds well to SCS. Patients with end-zone pain after spinal cord injury and those with intercostal neuralgias may benefit from SCS as well.
However the main group of patients considered to be candidates for SCS are those with a combination of neuropathic and nociceptive pain, a condition that is usually referred to as "failed back surgery syndrome.” Practical experience suggests that patients with predominantly radicular pain radiating to one or both legs respond better to SCS than those with predominantly axial low back pain.
A separate set of indications consists of pain syndromes resulting from tissue ischemia. Two conditions—lower extremity pain caused by occlusive vascular disease and intractable angina—appear to respond so well to SCS that they have become the primary indications for SCS in Europe. Interestingly, SCS does not mask the pain of acute myocardial infarction, making this therapeutic modality safer [7].
Table 1 Indications for Spinal Cord Stimulation
|
Common indications |
|
Failed back surgery syndrome |
|
Chronic arachnoiditis |
|
Painful peripheral neuropathy |
|
Postamputation stump pain |
|
Complex regional pain syndromes (types 1 and 2) |
|
Radiculopathy Spinal cord injury pain |
|
Questionable indications |
|
Intercostal neuralgia |
|
Phantom limb pain Additional indications Peripheral vascular disease |
|
Intractable angina |
In regard to the general selection of patients for SCS, the basic rules of pain surgery apply: patients should have failed less invasive therapeutic approaches and should have favorable psychological evaluations (ruling out somatization, major depression, and other psychological and psychiatric abnormalities). Also, in most cases one would prefer to know the exact diagnosis and underlying medical problem, although this may not be possible in some patients (eg, CRPS type 1). General contraindications to surgery or implantation of hardware, such as generalized infection, coagulation abnormalities, and serious concurrent medical diseases that would prevent even the brief general anesthesia necessary for the system implantation, need to be considered before the operation. Relative contraindications, such as advanced age, relatively short life expectancy, history of drug abuse, and the presence of other implanted electronic devices (eg, pacemakers), should also be taken into consideration. A separate condition for SCS surgery is the patient’s ability to perceive paresthesias in the painful area; this limits SCS use in patients with painful anesthesia. The final decision about permanent implantation of the SCS system depends on the results of the SCS trial; this will be further discussed in this topic.
Technical Considerations
Spinal cord stimulation technology has evolved from the relatively simple monopolar electrodes used in the original studies during the mid-1960s [8,9] to sophisticated multielectrode arrays for monopolar, bipolar, and tripolar stimulation that may be attached either to a completely implantable system for impulse generation or to a radiofrequency-coupled system with an im-plantable receiver and externally attached antenna controlled by a battery-powered impulse generator (Table 2).
The configuration of the electrode determines the ability to vary stimulation parameters, the position of the active electrode, and the direction of the stimulation. Therefore, more contacts are generally better, allowing more freedom in the selection of stimulation paradigms. However, the programming process may become cumbersome, and standard implantable generators may not be able to handle complex electrode configurations, which raises the second (and opposing) principle of "the simpler the better.” In most cases, the choice between the somewhat simpler, easier-to-use quad-ripolar electrodes and the more complicated, but also more versatile, 8- or 16-contact leads depends on each particular patient’s symptoms, pain distribution and patterns, and the results of prior treatments with SCS.
Table 2 Spinal Cord Stimulation System Components
|
Electrodes |
|
Percutaneous |
|
Four-contact leads |
|
Verify (temporary) (Medtronic 3862) |
|
Pisces-Quad (Medtronic 3487A) |
|
Pisces-Quad Compact (Medtronic 3887) |
|
Pisces-Quad Plus (Medtronic 3888) |
|
Quatrode (ANS) Eight-contact leads |
|
Octad (Medtronic 3898) |
|
Octrode (ANS) |
|
Laminectomy (paddle) electrodes |
|
Four-contact leads |
|
Resume II (Medtronic 3587A) |
|
Resume TL (Medtronic 3986) |
|
TTL lead (Medtronic) |
|
SymMix (Medtronic 3982) |
|
Eight-contact leads |
|
Lamitrode-8 (ANS) |
|
Specify (Medtronic 3988) |
|
Sixteen-contact electrodes |
|
Lamitrode-88 (ANS) |
|
Implantable impulse generators |
|
ITREL-II (Medtronic 7424) |
|
ITREL-3 (Medtronic 7425) |
|
Genesis (ANS) |
|
Synergy (Medtronic 7427) |
|
Synergy Versitrel (Medtronic 7427V) Radiofrequency receivers |
|
Renew (ANS, MNR908/MNR916) |
|
X-trel (Medtronic 3470) |
|
Mattrix (Medtronic 3271/3272) |
Selection of electrode type may also be affected by prior surgical interventions and individual anatomical variations. Percutaneous electrodes are easier to insert, but they have a greater tendency to migrate in the epidural space. Laminectomy (paddle or plate) electrodes require a larger surgical opening and allow less freedom in axial placement, but their rate of displacement is definitely smaller compared to that of the percutaneous (wire) electrodes. In addition to that, they seem to require less energy to achieve the same results as the wire electrodes, as their metal contacts face only the dura and are isolated from the posterior epidural space, whereas percutaneous electrodes are in circumferential contact with the surrounding tissues.
When it comes to choosing the power supply and programming equipment, here again each option has its own pitfalls and benefits. Fully im-plantable devices [implantable pulse generators (IPG)] are more convenient for patients because the entire SCS system is placed inside the patient’s body and the need for external attachments is eliminated. Patients can swim or shower without stopping the stimulation and do not have to worry about poor contact between the antenna and receiver. Implantable pulse generator systems, however, have only limited internal battery power, and, therefore, must be replaced every several (1-7) years, depending on the system usage and stimulation parameters. This obviously increases the long-term cost of the hardware. Also, the currently available IPGs have only limited options for electrode configurations and generally provide only single-channel stimulation. Patients with IPGs have fewer options for adjusting their stimulation parameters; however, this feature makes the devices somewhat safer.
Radiofrequency (RF)-coupled devices may, at least theoretically, serve forever without additional surgical interventions. The receiver is implanted subcutaneously and connected to the electrode(s). The power source/programming module is usually worn externally and communicates with the receiver through an externally applied flexible pancake-shaped antenna that is placed over the receiver. The battery change process is extremely simple, and the programming module is significantly more versatile than that of an IPG. In addition to the ability to change some or all of the stimulation parameters, some modules have integrated computer chips that memorize certain electrode configurations and stimulation paradigms and change from one to another with a simple push of a button. Most RF-coupled systems allow operations with two or more independent channels and are capable of covering 4, 8, or 16 electrode contacts. This becomes especially important in patients with complex pain patterns and in those cases where pain areas change with time. On the other hand, RF-coupled systems require a significantly higher degree of patient participation, which may be difficult for some patients. In addition, some patients develop dermatitis or other local skin reactions that prevent them from wearing the antennas for extended periods. Also, some patients state that having a permanent external device limits their freedom, and the are often willing to trade some of the benefits of RF-coupled systems for a completely implantable system.
Over the next few decades, technological advances will allow us to overcome most of these pitfalls. The SCS system of the future will combine all of the benefits mentioned here in one smaller and more versatile unit.
SCS Trial
Once the decision of whether to proceed with SCS is made, the surgeon must determine the type of the electrode and length of the SCS trial. There are two general approaches to SCS trials, each with its own advantages.
The first route assumes that the lead used for the trial will have to be discarded, regardless of the trial results. The trial starts with insertion of the screening electrode (which is usually less expensive but otherwise very similar to a permanent percutaneous electrode). The insertion does not require an incision and can be done in the doctor’s office; the lead is removed and discarded before the final implantation. An X-ray image of the temporary electrode is usually taken before its removal so the landmarks for permanent electrode insertion can be identified. This decreases the infection rate. The procedure can be done on an outpatient basis with a minute amount of local anesthetic, but there are some obvious drawbacks. First, there is the cost of the discarded electrode. Second, one has to enter the epidural space twice, even if the first electrode was placed perfectly and provided excellent pain relief. In addition, the surgeon must be confident that the second electrode will be inserted into the same position as the first, allowing a comparable degree of pain relief without an additional trial (as the second part of the surgery is usually done under general anesthesia). The idea of "screening” is, however, attractive, and many surgeons use this technique to evaluate the general responsiveness of the patient to SCS or as a prelude to the insertion of a laminectomy ("surgical”) lead.
Another approach assumes that the electrode used for the trial will subsequently be connected to the rest of the SCS system. The initial insertion of the electrode is performed so that it can be internalized, thereby eliminating the need for reinsertion. To minimize the risk of infection, the electrode is connected to a temporary extension wire, which in turn is tunneled under the skin and brought to the surface a few inches from the original incision. This extension wire is subsequently discarded, whereas the electrode itself stays in place and is connected to the permanent extension wire during internalization. To minimize the risk of displacement, the electrode is anchored to the fascia with nonabsorbable sutures. A radiographic image is obtained after the initial insertion and then again during internalization to rule out inadvertent electrode displacement. An obvious advantage of this method is that the final electrode position is tested before system internali-zation, allowing the second stage of surgery (IPG/receiver implantation and passage of the long extension wires) to be done under general anesthesia. On the other hand, the initial insertion has to be done in the operating room and if the trial fails, the patient has to be returned to the operating room to have the electrode removed.
Electrode Implantation Technique
The procedure of electrode implantation for SCS is fairly straightforward. Percutaneous (wire) electrodes are inserted through a needle. A standard 18-Ga Tuohy needle is supplied with the electrode and comes with a stylet. The needle is inserted into the epidural space under fluoroscopic guidance after the tissues are infiltrated with a local anesthetic solution. Usually, a slightly paramedian entry point is preferred to avoid midline placement of the fragile electrode, where it may be damaged by the hard spinous processes. The aim of needle insertion is usually a point just below the spinous process one or two levels cephalad to the point of skin penetration. This allows the electrode to be placed in the central posterior epidural space and guides the needle at about 5° to the skin surface, making the electrode passage somewhat easier. Entrance into the epidural space is best confirmed by the well-known "loss-of-resistance” technique: resistance against injection of air or sterile water through a glass syringe suddenly disappears when the needle penetrates the ligamentum flavum and enters the epidural space.
The level of electrode placement is chosen preoperatively based on the patient’s pain distribution. Previous experience shows that maximal paresthesias from SCS follow a somatotopic distribution. Positioning the active electrode at the T11-12 level results in paresthesias in the foot; T10-11, the anterior thigh; T4-5, the abdomen; C7-T1, the upper chest; and C4-6, the forearm and hand [10,11]. The low back region is probably the least responsive to SCS; the optimal position of the electrode to produce paresthesias in this region is usually at the T8-11 vertebral level [11].
Once the epidural space has been entered, the percutaneous electrode is introduced through the needle and then advanced in a cephalad direction under fluoroscopic guidance (Fig. 1). The electrode becomes relatively rigid with the guidewire inside; it is possible to manipulate the electrode inside the epidural space and advance it up to the desired level by gently pushing and rotating the electrode shaft. A slight curve of the guidewire tip allows minimal steering, which is especially important when passing through ste-notic levels or epidural scar tissue. It is generally advisable to place the percutaneous electrode one level higher than the final target and then optimize the electrode position based on the intraoperative trial by pulling the electrode down.
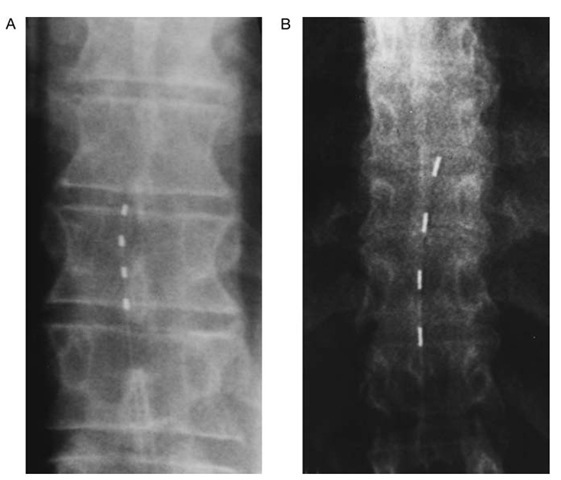
Figure 1 Intraoperative images of percutaneously inserted spinal cord stimulation electrodes. Each electrode has four contacts that may provide stimulation with a variety of configurations, including monopolar and bipolar stimulation modes. A, Pisces-Quad electrode (Medtronic); B, Pisces-Quad Plus (Medtronic).
If the subarachnoid space is entered during needle manipulation (manifested by cerebrospinal fluid outflow from the needle hub), the procedure does not need to be aborted. Most surgeons prefer to withdraw the needle and change the level of insertion so the electrode does not accidentally enter the dura through the hole made by the needle. In these cases, the patient should be warned of the possibility of spinal headaches and advised to stay flat for several hours and consume extra fluids, but the need for additional intervention, such as epidural blood patching, is rare.
The goal of the intraoperative trial is not to eliminate the pain nor to quantify the degree of pain relief, but to test the location of paresthesias and adjust the electrode position for optimal coverage. The electrode is connected to the screening device either directly or through a temporary extension wire, and the various electrode configurations are tested while the patient is asked about his/her sensations. The use of multiple contacts allows one to move the active (negative) contact along the electrode without actually repositioning the device. Stimulation-induced paresthesias follow the general somatotopic map; induction of paresthesias superior (cephalad) to the desired area requires repositioning of the electrode more inferiorly (caudal) and vice versa. It may also be necessary to shift the laterality of the electrode depending on the patient’s pain patterns and paresthesia thresholds. This is usually accomplished by carefully pulling the electrode one or two levels down and then advancing it with some rotational steering using the guidewire curvature. Obviously, when it comes to axial advancement, the percutaneous electrodes provide much more freedom to manipulate, with their flexibility and ease of insertion up to six or seven levels from the target location.
Once the intraoperative trial is completed, a fluoroscopic image of the electrode position is obtained and saved on the screen so it may be later compared with the final electrode position. The electrode is then anchored to the overlying fascia. This is done through small incisions above and below the point of needle insertion. The needle itself is removed after the incision is made but before the anchoring procedure. The electrode kit usually contains several anchors; some of them are soft and flexible, with lower profiles and less tensile strength. Others are made of hard plastic; these have higher profiles, but are more reliable for holding the electrode in place. It is advisable to place the anchor as close as possible to the point where the electrode passes through the fascia so that movement of the electrode is minimized. After that, a temporary extension wire is passed subcutaneously and brought to the skin surface 10 to 15 cm from the original incision. The entire path of the extension wire and the site of its exit through the skin is infiltrated with local anesthetic. Advance consideration of the final position of the implanted system allows the surgeon to tunnel the temporary wires on the side opposite the final position of the IPG/receiver, so the track of the permanent extension wires does not cross that of the temporary ones. The connection between the electrode and the temporary extension wire is then secured with all four screws and covered with a silicone sleeve. If this connection is placed close to the midline, it is easy to identify during the internalization procedure. The excess wire is usually coiled above the fascia but below the connection with the temporary wire to protect the electrode during internalization or revision.
The incision is then irrigated with antibiotic solution and closed in standard fashion. It is not necessary to place an additional suture at the site where the temporary wire exits. Coiling of the wire on the skin surface and adhesive dressing may be sufficient to prevent dislodgement, but one may still want to add a purse-string stitch to minimize the risk of infection. In general, however, long tunneling of the temporary wire, short duration (1 week or less) of the outpatient trial, and careful observation of sterile technique during all stages of surgery may lower the infection rate to zero. The general rules for electrode handling are listed in Table 3.
In some patients who have undergone prior surgical interventions and have extensive epidural fibrosis or a multilevel posterior fusion, it may be very difficult to enter the epidural space or manipulate inside it. In these instances, the open approach—surgical exposure of the epidural space and insertion of laminectomy electrodes—may be the only choice even at the trial stage.
The procedure for laminectomy electrode insertion is similar to the percutaneous placement, but the opening of the epidural space is done by a limited laminectomy immediately below the target level for stimulation. The generally accepted approach involves a midline opening of the spinal canal with removal of the spinous process, visualization and transection of the ligamentum flavum, and identification of the underlying dura mater. A special plastic template and curvilinear spacer are used to dissect the epidural plane and prepare the area for electrode insertion. Once again, intraoperative fluoroscopy is used to monitor electrode advancement and position (Fig. 2). It is also possible to insert the laminectomy-type electrodes through a unilateral spinal exposure. With this method, a small hemilaminotomy is per- formed immediately lateral to the spinous process, and the root of the spi-nous process is undercut with rongeurs. This approach minimizes surgical trauma and is better tolerated by the patients, which is particularly important as most procedures are done under local anesthesia.
Table 3 Considerations for SCS Lead Implantation
|
• |
Avoid bending or kinking the lead. If there is any excess lead body, create gently coiled loops of no less than 2 cm. |
|
• |
Use fingers or a rubber-tipped bayonet forceps when handling the lead. |
|
• |
Do not tie a suture directly to the lead body. Use the anchor supplied in the lead kit. Avoid placing tension on the lead during surgery. Leave the lead body as loose as possible after connecting the extension to avoid unnecessary tension on the lead. |
|
• |
Do not force the lead up the epidural space. Use the guidewire packaged in the lead kit to clear a path for the lead. |
|
• |
Be extremely careful with sharp instruments around the lead. |
|
Should any system component become damaged during implant, remove it and replace it with another component for permanent system implantation. |
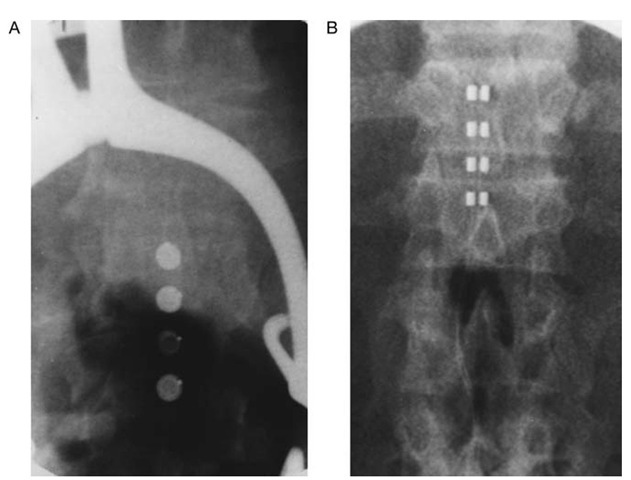
Figure 2 Intraoperative images of surgically implanted laminectomy electrodes (courtesy of Dr. Kim J. Burchiel, Portland, Oregon). A, Resume four-contact electrode (Medtronic). B, Specify eight-contact electrode (Medtronic). Specify electrode allows for either simultaneous stimulation with identical configuration of contacts using an implantable generator or independent stimulation with different configuration of contacts using radiofrequency-coupled systems.
The surgical lead may be placed either above or below the laminec-tomy/laminotomy level and the position of the lead is adjusted based on the intraoperative trial results. The procedure for anchoring and tunneling the surgical lead is essentially identical to that for percutaneous electrodes, except in some cases the lead may be anchored to the interspinous ligament before layer-by-layer closure of the incision. It is important to note that sometimes surgical leads may be hard to remove, especially after they have been in the epidural space for a long time, and therefore it may be necessary to remove the wire but leave the paddle inside.