Introduction
Spasticity arises from a variety of neurologic disorders, including cerebral palsy, multiple sclerosis, cerebrovascular accidents, spinal cord injury, and head trauma.
Selective posterior rhizotomy is now an accepted procedure for relieving spasticity in carefully selected patients with spastic cerebral palsy (CP). Recent developments in electrophysiologic monitoring and refinements in surgical technique have led to a resurgence in the use of this procedure for patients with spastic cerebral palsy [1-7]. Dorsal rhizotomy is performed through bilateral L2 to S1 laminectomies or laminotomies to allow selective division of lumbosacral posterior spinal rootlets with electromyographic (EMG) guidance. In patients with cerebral palsy, judicious patient selection, intraoperative monitoring, and intensive postoperative physical and occupational therapy are essential for successful surgical outcome [1,8]. Ambulatory patients with spastic diplegia and those with pure spasticity without motor weakness or severe contractures show the greatest functional improvement [1,8,9].
Spasticity and Cerebral Palsy
Spasticity in childhood is most commonly encountered as a feature of cerebral palsy, which results from an insult to the developing brain. Cerebral palsy may be classified by the type of motor involvement (spastic, dystonic, or mixed), or by the distribution of involvement (quadriplegia, diplegia, hemiplegia). Loss of fine motor control, impaired balance, weakness, and delayed motor milestones also occur. The progressive, deforming forces of spasticity can lead to secondary muscle contractures and orthopedic deformities, such as hip dislocation and scoliosis [10,11]. This disorder varies widely in severity and may be associated with other problems such as hy-drocephalus, seizures, learning disabilities, language problems, or sensory processing disturbances. Developmental delay is often but not always a feature of CP. Spasticity in children is particularly problematic because of the interference with normal growth.
Patient Selection
When considering the goal of neurosurgical intervention, spastic cerebral palsy patients can be divided into two groups. The first group is composed of those patients in whom functional improvement is expected. An example would be a spastic diplegic 5 year old who walks independently with a scissoring gait, flexed hips and knees, and an equinus foot posture. There should be no evidence of dystonia and minimal fixed contractures. Although range of motion may be limited in straight leg raising because of tight hamstrings, in hip abduction because of tight adductors, and in ankle dor-siflexion because of tight gastrocnemius-soleus muscles, a large portion of the restricted movement may be caused by dynamic tightness rather than structural shortening. By reducing spasticity, range of movement and stride length should increase, posture should improve, and walking speed may be accelerated. The second group is composed of those nonfunctional, severely affected spastic quadriplegic patients in whom functional gains are unlikely, but reduction of spasticity is expected to improve patient comfort, ease patient care, and reduce the risk of developing contractures, bony deformities, and joint dislocations.
Rhizotomy is not effective in patients with severe, fixed contractures. Tendon-lengthening procedures of structurally shortened muscle groups such as hip adductors, gastrocnemius-soleus, and hamstring muscle groups by the orthopedic surgeon may be indicated. In cases in which the patient has already undergone tendon-lengthening procedures, rhizotomy is often con-traindicated, as a reduction in tone may adversely affect posture.
Assessment of strength and control of voluntary movements are essential. Some patients have little voluntary control and rely on the increase in tone to maintain posture. For them, reducing spasticity may actually be detrimental. Identifying certain features can help in the difficult differentiation between spastic patterns and voluntary movement. A voluntarily controlled movement can be initiated and halted several times throughout its range, whereas spastic movements tend to occur in gross patterns that, once initiated, cannot be interrupted. If adequate voluntary power cannot be confirmed, then selective posterior rhizotomy is contraindicated.
Other factors to evaluate are truncal control, isolated muscle control and the presence of primitive reflexes. If the goal is to improve gait and truncal control is poor, then reduction of spasticity in the lower extremities is unlikely to help. In severely affected spastic, quadriplegic children, rhi-zotomy is unlikely to improve function, but it may help to prevent contrac-tures or aid in positioning and posture.
The role of spasticity relative to the degree of clinical disability should be considered. If weakness is minimally present and spasticity predominates, selective dorsal rhizotomy may improve function. The procedure is contra-indicated in patients with significant weakness (particularly postural muscles), extrapyramidal disorders such as dystonia, athetosis, or rigidity, poor truncal control, overlengthened tendons, severe contractures, or fixed spinal deformities.
Operative Technique
Intact neuromuscular activity is essential for intraoperative monitoring; therefore, no neuromuscular blocking agents are used during the procedure after the laminectomy or laminotomy has been performed. If excessive muscular response to posterior nerve rootlet stimulation is encountered, deepening the level of anesthesia with inhaled agents decreases the abnormal excitability, preventing overt movements and facilitating the electrophysio-logical evaluation.
After induction of general anesthesia and placement of an indwelling Foley urinary catheter, the patient is placed prone on the operating table with bolsters under the chest and pelvis to enable the abdominal wall to move freely. This facilitates respiratory movements and prevents elevated venous pressure in the epidural veins and thus minimizes blood loss. The knees, feet, and elbows are supported and padded carefully with soft foam. The patient is positioned with the feet near the lower end of the table, with the head turned to the side on a soft circular headrest. The intercristal line between the posterior iliac crests is used as a reference to locate the level of the fourth lumbar spinous process. Alternatively, a cross-table lateral radiograph may be used for localization. Counting from the L4 spinous process, the skin is marked from L1 to S2. Local anesthetic is injected through the marked incision. After careful skin preparation, the surgical drapes are arranged over a Mayo stand positioned above the patient’s feet and legs to provide access for the EMG team. A transparent drape can be used to allow visualization of the lower extremities by the surgeon. The EMG team places needle electrodes into the muscle groups of interest at this time. Standard electrode placement includes five muscle groups (hip adductors, quadriceps, tibialis anterior, hamstring, and gastrocnemius) and the external anal sphincter muscle bilaterally.
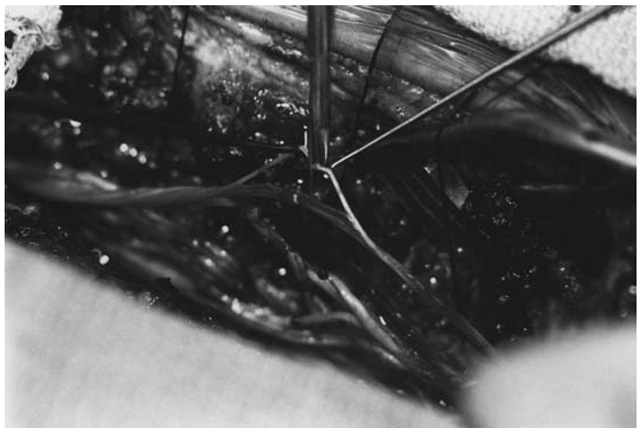
After making the midline skin incision, self-retaining retractors are placed. The lumbodorsal fascia is incised on either side of the supraspinous ligament, which is preserved. The paraspinal muscles from L1 to S1 are retracted laterally using subperiosteal dissection. The lowest mobile spinous process is identified, usually L5, but in young children may be S1. The ligamentum flavum is incised with a No. 15 blade below the laminae of L5 to expose the epidural fat. A laminectomy may be performed, but in most cases a laminotomy from L5 to L2 is preferred. The laminae from L5 to L2 are cut bilaterally with a high-speed drill with a foot-plate attachment. The facet complexes are carefully preserved and the width of the laminotomy need not exceed 10 mm. The supraspinous and interspinous ligaments are divided between L5 and S1 and the entire segment is retracted rostrally, hinged on the interspinous ligaments between L1 and L2. In similar fashion, the laminae of S1 are cut and the S1 segment is retracted and secured cau-dally, thereby preserving the supraspinous ligament and exposing the upper sacral dura (Figure 1). Hemostasis is achieved by applying bone wax to the bone edges and bipolar cautery to epidural veins, and the epidural fat is cleared away to expose the dura. The dura is incised using a fresh No. 15 blade, preserving the arachnoid membrane. The dural incision is extended cephalad and caudad using a grooved director and the No. 15 blade. The dural edges are tacked laterally with fine suture. The arachnoid membrane is then incised, exposing the cauda equina. Cerebrospinal fluid is aspirated through a cottonoid to protect the nerve roots.
Attention is then directed toward accurate identification of the nerve root level by stimulating the anterior roots of S1 and S2. Two specially insulated rhizotomy electrodes with blunt hooks (Aesculap Surgical Instruments, Burlingame, CA) are connected by sterile wires to the electrical stimulator. The S1 nerve root, which is usually the largest in the cauda equina, is isolated and by gentle manipulation, the cleft between its anterior and posterior roots is identified. The posterior root is broad and flat, while the anterior root is round and smaller (Figure 2). The anterior root of S1 is stimulated, and characteristic flexion at the knee and plantar-flexion at the ankle are observed. Similarly, stimulation of the anterior root of S2 should produce plantar-flexion at the ankle and flexion at the toes. At this point, the anatomical levels have been identified by using (1) bony landmarks, (2) the size of the S1 root, and (3) the motor responses from the anterior roots of S1 and S2.
Figure 1 Exposure for selective posterior rhizotomy. The L5 to S1 segment has been reflected rostrally, and the S1 segment is reflected cau-dally. This preserves some blood flow to the posterior elements and provides for rapid and efficient bony closure.
Figure 2 Separation of a spinal root into its round anterior root, and broad, flat posterior root.
Counting cephalad, the L2 root is identified and separated into its anterior and posterior components. The posterior root is then picked up with the blunt hook electrodes and initially stimulated with a single 0.1 msec stimulus, which is increased incrementally until the threshold for muscular contraction is reached. The rootlets comprising the posterior root of L2 are then carefully separated and stimulated. After a threshold muscular contraction to a single-pulse stimulus is determined, each individual rootlet is stimulated with a 1-second-duration subthreshold 50 Hz tetanic stimulus, and the muscular response is monitored. The selection of rootlets for division or preservation is discussed in greater detail in the following section on intra-operative monitoring. The assistant gently retracts the rootlets associated with a normal response on a separate noninsulated nerve hook. Those rootlets associated with an abnormal response are divided using neurosurgical microscissors (Figure 3). The stimulation and recording are repeated at each level from L2 down to S2. The surgeon works with the rootlets on the contralateral side and moves to the other side of the table to repeat the procedure when the first side is completed. In a typical rhizotomy procedure, between 50 and 75 rootlets are stimulated and, depending on the degree of spasticity, between 25% and 50% are subsequently divided.
Throughout the procedure, accumulated cerebrospinal fluid is aspirated through a small cottonoid. There are typically between two and four posterior rootlets at the L2 level, with an increasing number at each subsequent level down to S1, where there are usually between 8 and 11 rootlets. The S2 root is the first to be smaller than the preceding root and usually contains two to six very fine rootlets. When identifying the S2 root, it is important to also visualize the S3 root, which may be adherent to the S2 root and inadvertently stimulated with an S2 rootlet and divided.
Figure 3 Microscissors are used to section a rootlet generating an abnormal response.
After completion of the stimulation and rhizotomy stage, the dura is closed with either a continuous locking or interrupted suture. Before placement of the last stitch, sterile preservative-free saline is injected into the intrathecal space with a blunt needle. The anesthesiologist performs a Val-salva maneuver and any sites of leakage are oversewn until a watertight dural closure is confirmed. The previously rotated posterior elements are brought back into position and the medial and lateral cut edges of the li-gamentum flavum are sutured at each level. Next the spinous process of S1 is sutured to that of L5 after replacing the S1 spinous process in its anatomical position. The paraspinous muscles are reapproximated through the in-terspinous ligament using interrupted suture. This brings the muscles into good alignment with the bone and ligaments before the supraspinous ligament is sutured to the lumbodorsal fascia. The skin is closed in two layers, the first with subcutaneous interrupted absorbable suture and the outer layer with a continuous locking nylon suture. A sterile, occlusive dressing is applied and the patient is taken to the recovery room or directly to the pediatric intensive care unit.
Intraoperative Monitoring
Rootlet selection for division is based on the electromyographic response to electrical stimulation and visible muscle contraction. The overall clinical picture is considered, and sacral-level rootlets associated with anal sphincter activity are spared. Recordings from the muscles are made from 2.5 cm. stainless-steel needles, placed two each in the anal sphincter and in five muscle groups of each leg: hip adductors, quadriceps, tibialis anterior, hamstrings, and gastrocnemius. This allows bipolar recording of each muscle group, with tracings displayed on an electroencephalography chart recorder at 30 mm/sec for simultaneous viewing of activity from all 11 muscle groups.
Sterile, insulated rhizotomy electrode hooks are used to stimulate the nerve roots with an electrical stimulator that allows delivery of both single pulses and trains of stimuli. A constant-voltage electrical stimulator is used because the voltage range remains fairly constant despite rather large differences in the cross-sectional area of rootlets, whereas the amount of current required would be greater for thicker rootlets than for thin ones. The roots may then be stimulated in a bipolar manner by gently lifting the root away from the cerebrospinal fluid and applying a stimulus. The stimulating contacts are separated by 5 to 10 mm, and the voltage is varied throughout the testing procedure. Typically, between 0.2 and 5 volts are required for anterior roots and 20 to 100 volts for posterior roots. The whole posterior root at one level is first stimulated with single pulses to identify its threshold, which usually corresponds to the rootlet threshold. Next, the root is subdivided into its rootlets, which are stimulated in turn. Initial stimulation for each rootlet uses single pulses, delivered individually at gradually increasing voltages until the threshold for muscle contraction is reached. Next, 1-second trains of stimuli are applied at voltages reduced to 30% to 50% of the single pulse threshold, and gradually increased until the threshold for muscle contraction is achieved. Next, at the train threshold, several repetitions are applied to evaluate the pattern of muscle contraction and to assure reproducibility of the findings for each rootlet. Suprathreshold stimulation is avoided because diffuse spread of muscular contraction is usually produced inappropriately and may be interpreted as an abnormal response.
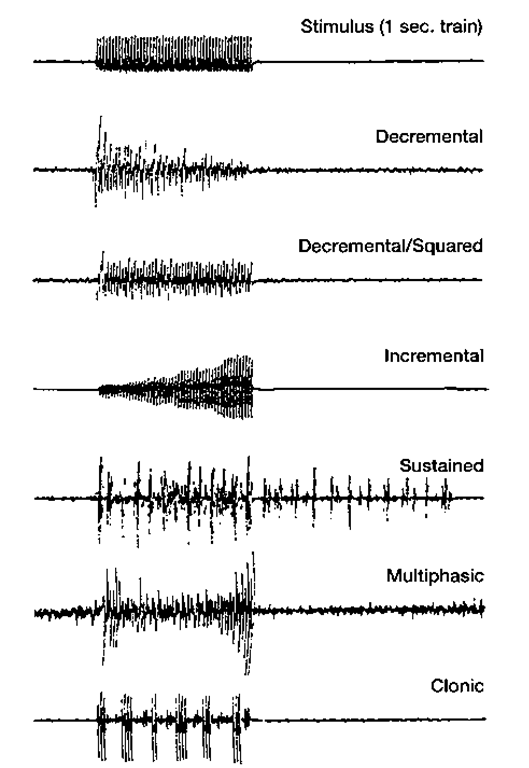
Several patterns of EMG response are seen during trains of stimulation (Fig. 4). "Squared” responses show a uniform amplitude of EMG across the entire 1-second interval of train stimulation. ”Decremental” responses either gradually decrease in amplitude across the 1-second interval or decrease mainly in the first 100 msec and remain squared thereafter. The squared and decremental responses are considered normal. "Incremental," ”multiphasic,” ”clonic,” "sustained," ”spread,” and ”contralateral spread” patterns are considered abnormal. The EMG amplitude of incremental responses rises abruptly or gradually during the train of stimuli. Clonic responses involve repeated bursts of EMG activity, often 5 to 12 bursts within a 1-second train. Multiphasic responses have several phases of incremental and decre-mental patterns within the same period of stimulation. Sustained responses show persistence of the EMG activity beyond the 1-second of 50 Hz stimulation. Equivocal responses fall outside the above categories, lying somewhere in between squared and clonic responses. Spread describes responses in which stimulation produces contraction of other muscle groups than the one being stimulated on the ipsilateral side, and contralateral spread refers to contraction of contralateral muscle groups (Figs. 5 and 6). Ongoing background muscle activity may be present and should be disregarded in the evaluation of responses. If excessive background firing is present or if higher voltages are needed to obtain responses, adjustments in the level of anesthesia may alleviate the problem.
Generally, between 25% and 50% of rootlets are cut, but slightly more may be divided in a severely affected patient. Division of all the rootlets at any level should be avoided, and a more conservative approach should be used in a root undergoing division of multiple consecutive rootlets (usually greater than three).
Figure 4 Examples of single-channel electromyogram recordings seen intraoperatively. The decremental and decremental-squared responses are considered normal, whereas the incremental, sustained, multiphasic, and clonic responses are abnormal.
At sacral levels, particularly S2, any activation of anal sphincter activity is a contraindication to sectioning of that rootlet, even with abnormal lower extremity responses. The decision to divide or spare a rootlet is based primarily on the EMG response pattern to trains of stimuli, but clinical judgment is also used. The behavior of the leg assessed visually or by palpation is considered, as are factors such as the number of rootlets previously cut at that level, the distribution and severity of the spasticity, and the functional level of the child.
Figure 5 Examples of ipsilateral and contralateral spread. Stimulation of an S1 rootlet on the right demonstrating proximal ipsilateral spread.