Introduction
The field of functional neurosurgery has developed hand in hand with that of stereotaxis. Although Meyer first performed ablation of basal ganglia structures for control of tremor in 1942 as an open procedure, the safety of these procedures was significantly improved by the development of the stereotactic apparatus [1]. A number of stereotactic frames (and atlases) have been developed, most notably by Spiegel and Wycis [2], Talairach [3], Leksell [4], and Brown, Roberts, and Wells [5]. Successful placement of percutaneous lesions and implants relies on the precise subcortical localization each of these devices affords.
A number of technological advances have further improved the accuracy and safety of stereotactic procedures. First have been the improvements in imaging techniques, evolving from ventriculography to three-dimensional (3D) computed tomography (CT) and magnetic resonance imaging (MRI).
At the same time, the development of faster microprocessors has enabled a symbiosis of advanced imaging techniques, computational technology, and stereotaxis, leading to the advent of computer-assisted image-guided stereotaxis. This topic will address the application of this technology to functional procedures for movement disorders.
Advantages of Computer-Assisted Image Guidance
Computer-assisted image guidance provides significant advantages for functional movement disorder procedures (Table 1). These advantages improve the accuracy of target localization by various methods (including anatomical formulaic, and atlas-registration techniques), and significantly decrease the number of steps and time required for target site calculation. The benefits of computer assistance at various stages of the procedures are best exemplified by a step-by-step analysis of the use of these techniques during a typical procedure for movement disorders, the implantation of a subthalamic nucleus (STN) deep brain stimulator for Parkinson’s disease.
Stereotactic Procedures For Movement Disorder Surgery
Frame Application and Fiducial Localization
Under laboratory conditions, "frameless" computer-assisted image guidance is capable of submillimeter targeting accuracy [6], but rigid frame fixation continues to be used for most functional intracranial neurosurgical procedures to provide the highest accuracy possible. Many stereotactic frame systems are currently in production. However, this discussion will focus on the Leksell G frame (Elekta Instruments, Atlanta, Georgia) used in conjunction with the StealthStation (Medtronic Sofamor Danek, Broomfield, Colorado), one of several commercially available computer-assisted image-guidance systems.
Table 1 Advantages of Using Computer-Assisted Image Guidance in Functional Neurosurgery
|
Rapid fiducial localization |
|
Referencial localization of every point in a three-dimensional volume |
|
Rapid correction of tilt, twist and rotation of head and frame within the scanner |
|
Rapid correction of tilt, twist and rotation of frame in relation to the ICL Rapid formulaic targeting based on distance from the ICL |
|
Ability to merge multiple data sets (such as different MRI sequences, fMRI, PET) |
|
Ability to superimpose (and morph) computer-based stereotactic atlas for target selection |
|
Ability to superimpose post-operative imaging on operative planning to check accuracy |
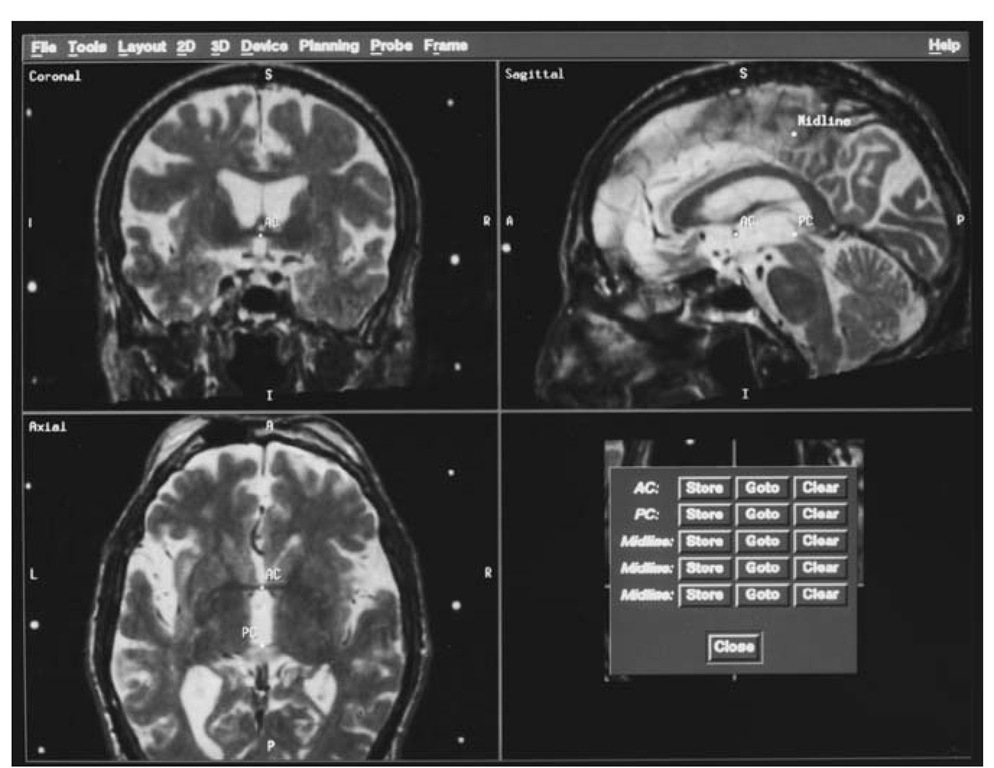
Figure 1 Screen from image-guided navigational workstation (Stealth, Medtronic Sofamor Danek, Broomfield, CO) showing the reformation of the volumetric data set parallel or orthogonal to the intercommissural line (AC-PC) and the vertical midline. AC, anterior commissure, PC, posterior commissure.
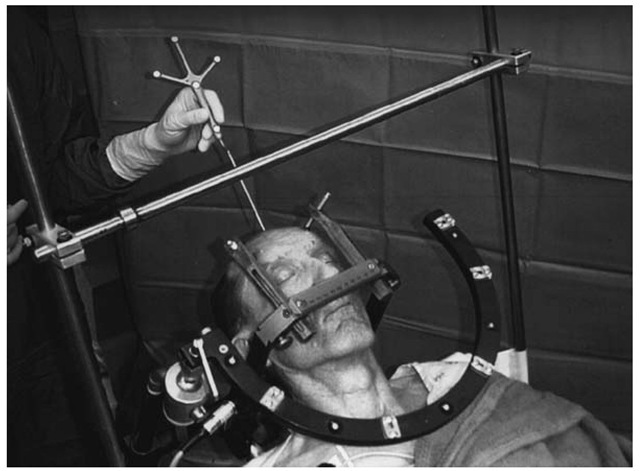
Frame-based stereotactic procedures first involve affixing the frame with four skull pins under local anesthetic. The frame base is fitted with a temporary fiducial-containing cage, the localizer. Imaging with CT, MRI (one or more sequences), magnetic resonance angiography (MRA), or ve-nography (MRV) is then undertaken with the frame resting rigidly on a holder. More than one dataset can be merged before fiducial localization to allow estimation and consideration of potential image distortions (as with MR), or to allow consideration of different types of information (parenchyma, vasculature, etc.) Computer workstations allow effortless shifting between datasets.
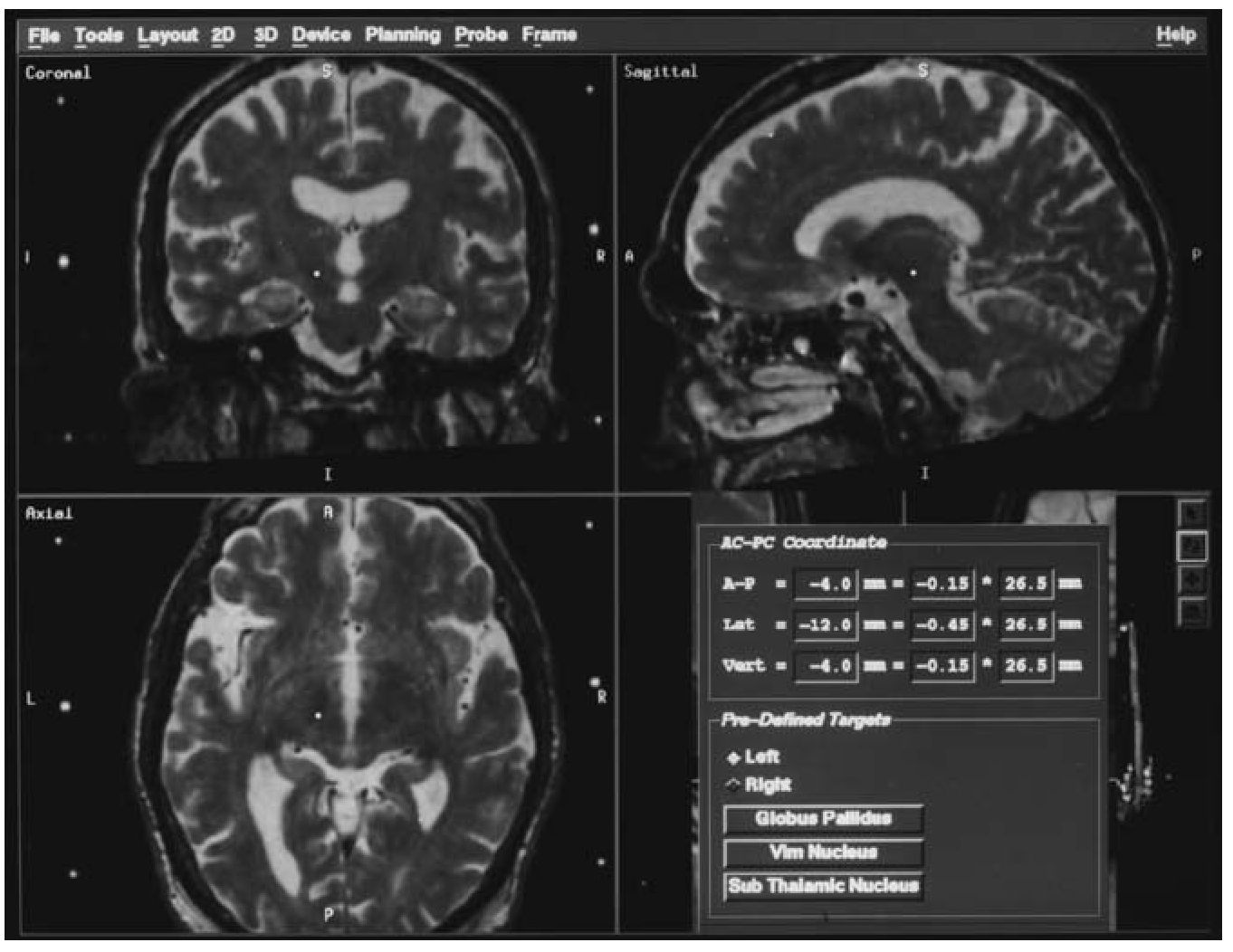
Figure 2 Screen from image-guided navigational workstation showing the positioning of a formulaically-calculated target for a pallidal procedure using the workstation software.
Previous techniques that did not use computer assistance required that the base ring be coplanar with the plane of the scanner gantry to maintain the geometry upon which target-centered stereotactic devices rely. This is not the case with computer-assisted techniques. The fiducials in any axial plane (not necessarily the one in which the target lies) are imported into the software using a cursor. Thereafter, the fiducials in each adjacent slice of the scan are automatically detected, generating a complete volumetric representation of the fiducial localizer. This registration allows the software to know the precise geometry of the localizer within the scanner, thereby eliminating the need to adjust the fiducial localizer position during scanning and leading to considerable economy of time.
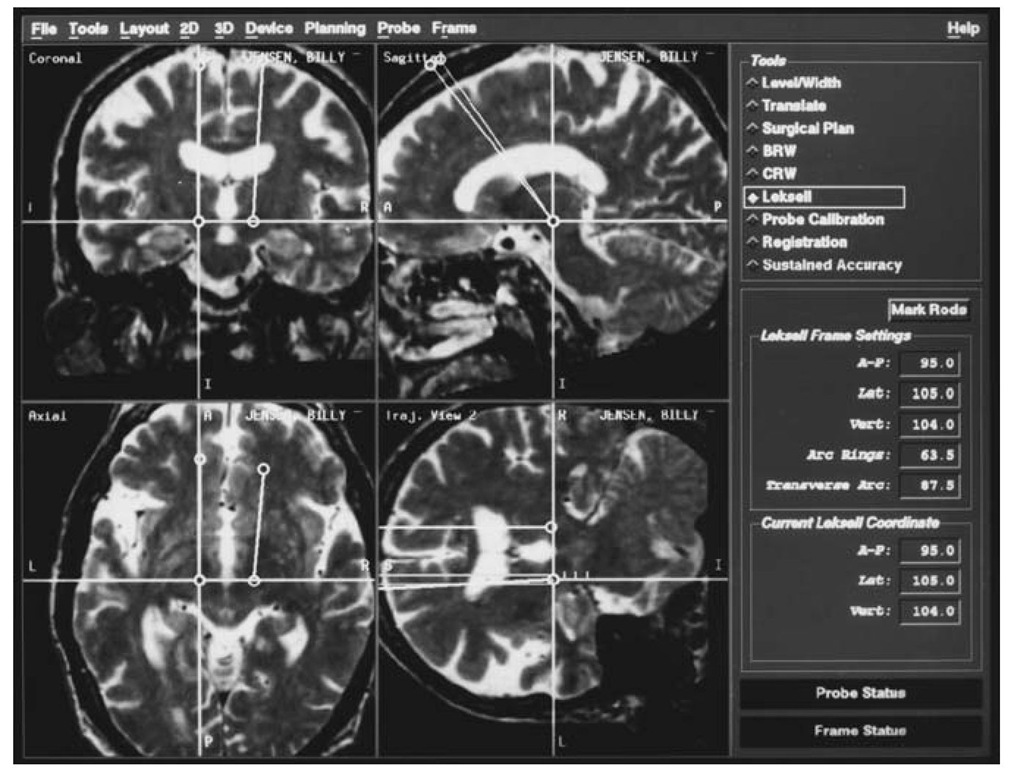
Figure 3 Screen from image-guided navigational workstation showing the use of trajectory views to view the course of an implanted electrode following trajectory planning. Only in the ”trajectory view” is the entire course of the electrode ”in plane.”
Target Localization
After fiducial localization, the potential stereotactic target is calculated. In many instances, the target is calculated in relation to the intercommissural line (ICL) using standard formulas as pioneered by Talairach [7]. Previously, calculations required that the base ring lie parallel to the ICL to prevent geometric errors in projecting distances perpendicular to the ICL (or the use of complicated geometric calculations). This was accomplished by careful placement of the frame on the patient’s head in relation to external landmarks or repositioning of the frame after a sagittal scout. Computer-assisted image guidance obviates this step by allowing reformation of the 3D imaging volume in a plane parallel to the ICL (Fig. 1). Once the anterior commissure and posterior commissure are marked, the image set is automatically corrected for tilt and rotation. The midline is manually identified at three points, and the software then automatically corrects for twist errors.
Figure 4 Following registration of the Leksell™ base ring to the arc of the Stealth™ frameless stereotactic system, using Framelink® software, the coordinates in frame-space of any point on the patients skull can be instantly determined. This might be used, for example, when you want to plan a trajectory that enters through a patient’s previous burr hole.
In movement disorder procedures, target identification usually takes place using either coordinates referential to the ICL, or through the use of coregistered brain atlases (Schaltenbrand and Wahren) [8]. The Stealth-Station software facilitates the use of either method. Formulaic target coordinates based on the ICL are preprogrammed, yet modifiable (Fig. 2). The correction of tilt, twist, and rotation of the ICL allows rapid adjustments in relation to the ICL, such as choosing a point that is at the same anterior/ posterior and vertical position as that generated by a formula, but that is more lateral or medial in relation to the ICL. Additionally, a fully deformable Schaltenbrand and Wahren atlas resides in the operating software for direct overlay upon axial images, if atlas coregistration is the preferred technique. Of course, a combination approach, or one incorporating direct anatomical targeting (as, for example, of the STN), is easily accommodated. Furthermore, as all axial images are available in the volumetric space, choice of multiple targets in different axial planes is easily done, even in the operating room.
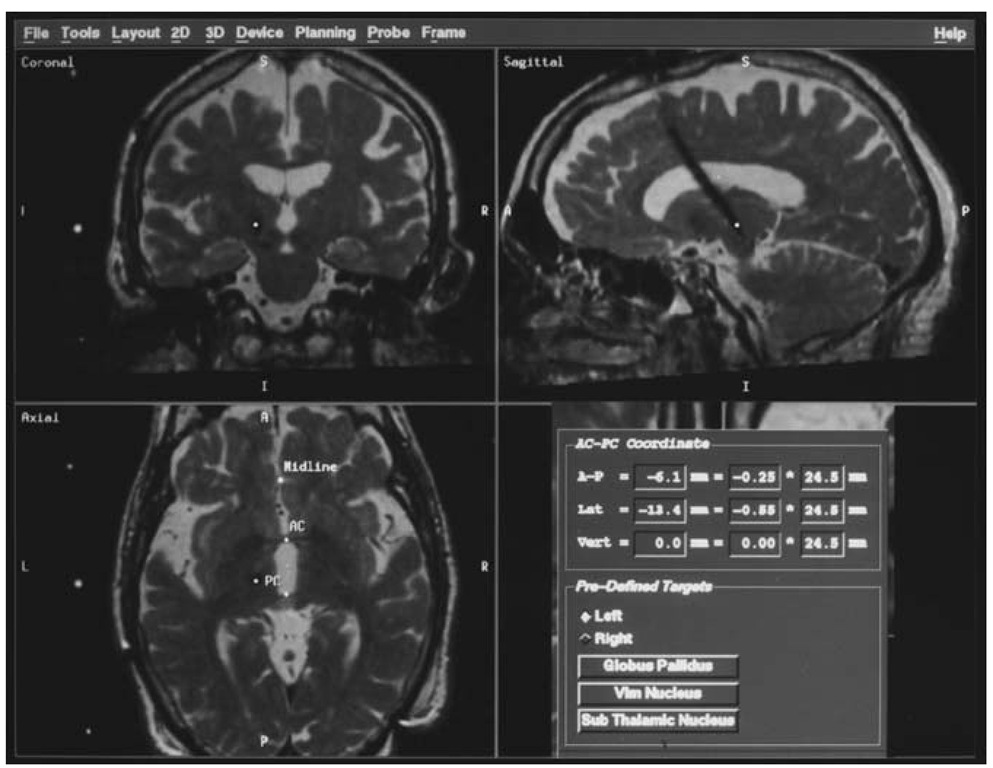
Figure 5 The use of the image-guided surgery software to determine the precise location of an implanted DBS lead postoperatively, in relation to the intercommissural line (lower right). In this case, rather than using the ”pre-defined targets” function, the cursor was positioned over the tip of the electrode, and the relationship of this point to the intercom-missural (AC-PC) line is calculated (under ”AC-PC coordinates”).
Trajectory Planning
Once a target is selected, entry points can be determined. Here again computer-assisted image guidance allows a great deal of flexibility in surgical planning. Entry points can be determined preoperatively based on constraints such as the location of sutures, blood vessels, sulci, and ventricles. Moreover, a trajectory that will lead to a particular orientation of a deep brain stimulation (DBS) lead within a target structure can be chosen (for example, double oblique orientation to match that of the STN). The pathway from the entry to the target can be examined in detail using the "planned pathway” or "probe’s eye view” options, allowing virtual surgery before the patient is taken to the operating room (Fig. 3).
Entry points constrained by pre-existing scars or burr holes can be determined in the operating room using the Framelink®, which allows the registration of the stereotactic frame to the optic digitizer. The stereotactic coordinates of the frameless pointer are instantly calculated and then easily used in trajectory planning (Fig. 4).
Intraoperative Use
Frame coordinates corresponding to the desired trajectories are instantly calculated and displayed, allowing trajectory revision to proceed quickly. The Framelink® feature allows the procession of a microelectrode to be tracked as it is introduced into the target structure and projected onto the preoper-ative image set. The results of micro- or macroelectrode recording and stimulation can be annotated onto the surgical plan to aid in choosing further trajectories. Mirror-imaging of a trajectory from one side to the other is also easily accomplished, as the reformatted dataset is parallel to the ICL. The final position of lesions or implanted deep brain stimulator leads can be saved for comparison to coregistered postoperative image sets (Fig. 5) to confirm the stereotactic accuracy of the implantation or lesion procedure.
Conclusions
Computer-assisted image-guidance systems have the ability to simplify and streamline surgical planning and execution, and accommodate a number of different approaches to stereotactic targeting in movement disorder surgery. The StealthStation is equipped with optical digitizers to allow "frameless" stereotaxy; however, we continue to use rigid frame fixation at our institution to achieve the highest accuracy possible. The StealthStation has reliably allowed safe trajectories to be planned and executed. Lesion and DBS electrode placement have been found to correlate well with planned target locations.