Analysis of Intact Sphingolipids by Mass Spectrometry
Mass spectrometry is a powerful detection technique that enables separation and characterization ofcompounds according to their mass-to-charge ratio (m/z). Its essential components include a sample inlet, ion source, mass analyzer, detector and data handling system. The combination of sensitivity, selectivity, speed and ability to provide invaluable structural information makes MS an ideal method for analysis of intact lipid molecular species.
The interest in the analysis of lipids in general especially SBs, SB1Ps, Cers, Cer1Ps, Glc/GalCers and SMs has continued to evolve due to the importance of these molecules in various biological transformations. SPL molecular species exist in nature as a complex mixture of closely related components which differ in the fatty acid chain length, degree on unsaturation and hydroxylation. These species differ greatly in their chemical and biological properties. Various analytical methods have been employed to separate and analyze individual species from intact (underivatized) form, out of which ESI/MS is the method of choice providing the following advantages:44
• Elimination of time consuming derivatization steps
• Making possible the study and follow-up of biosynthesis, metabolism, turnover and transport of the molecular species
• Protect possible rearrangement of the fatty acid chain during derivatization.
The sample introduction can be either by direct infusion or through preceding separation devices such as liquid chromatography (LC).
Mechanism of Electrospray Ionization Mass Spectrometry (ESI/MS)
ESI/MS, invented in the 1960s, was put into practice by Fenn et al.45,46 It involves transformation of ions from the liquid to the gas phase. It is a method that operates at atmospheric pressure and ambient temperature. Initially, a solution containing the analytes of interest is introduced to the ESI ion source through capillary tubing. The narrow orifice at the end of the capillary and the dynamic forces facilitate formation ofsprayed small droplets in the ionization chamber. Application ofelectric potential (approximately 2-5 kV) causes ionization, consequently the droplets carry a net charge. The charged droplets are then directed into the mass analyzer by the applied electric field. The applied potential may be positive or negative depending on physicochemical properties of the analytes. Passing through the ionization chamber, the droplets dissolve and this effect dramatically increases the columbic forces between the ions. Once this force exceeds the surface tension of the solvent, the droplets explode to form a fine mist of smaller droplets. This cycle is repeated until molecular ions are generated prior to their entrance into the mass analyzer.
The soft ionization can generate lipid molecular ions without causing extensive fragmentation .18-25-48-50
MS Scan Modes
A number of mass analyzers are available, e.g., quadruple, ion trap, time of flight, ion cyclotron resonance, or sector instruments, which separate charged molecules in vacuum depending on their m/z ratio.
In so called full scan (FS) mode, a spectrum of primary, mostly molecular, ions is identified. This is the least specific mode with low sensitivity and it is mostly used for a rough assessment of major components of biological material when no or very limited information about SPL composition exist. However, interferences from other compounds present can either suppress ionization or cause a high chemical noise making such detection of SPL virtually impossible.
Moreover, the mass analyzers can also be used for fragmentation, predominantly in the triple quadruple instrument. In this instrument, the middle (Q2) field free quadruple either focuses and transmits all ions, or can be used as a collision cell for controlled fragmentation, called collision induced dissociation (CID). As results of a collision with an inert gas, introduced into the collision cell, the internal energy of the ions increases through conversion of kinetic energy breaking out specific bonds, depending on the collision energy applied.51-52 The fragment ions are then analyzed in the second mass analyzer (third quadruple Q3). The choice of collision gas, its pressure and particularly the applied collision polarization and energy, affect the degree of fragmentation.
When a single quadruple instrument is used, partial fragmentation can be induced in the source by elevating the cone-to-skimmer potential difference. Protonated molecules desorbed from the ESI droplets are accelerated between the cone and skimmer, undergoing CID upon collision with residual carrier gas molecules.
Initial "soft" ionization of extracts prepared from biological samples results in numerous SPL molecular ions either positive (M + H)+ or negative (M-H)~. When the precursor ion fragments, it generates secondary (called daughter) distinctive pattern of ions related to the head group, SBs and fatty acids. This provides a wealth of structural information, enabling identification of SPLs in particular biological material. The positive ionization fragmentation can be enforced by incorporation of alkali metal ions (M + Me)+ where Me = Li+, Na+, K+, Rb+, or Cs+.18,53 In addition to structural information, tandem MS provides a higher sensitivity, specificity and greatly reduced chemical background, thanks to very selected mode of monitored masses.
Specific Scan Modes for MS/MS Instrumentation
Product Ion Scan
In product ion scan, the first mass analyzer (Q1) allows a single ion with a set m/z value to pass and this is then further fragmented by CID in the second quadruple (Q2), the secondary (daughter) ions are then scanned over a defined mass range by the third quadruple (Q3) and passed to the detector. The relative abundance of the product ions depends on the dissociation dynamic; therefore, changing the CID collision energy, a fragmentation pattern is observed which is specific for each SPL class of compounds.
Neutral Loss (NL)
In a neutral loss scan, Q3 is offset from Q1 by fixed m/z, corresponding to specific neutral loss, e.g., 18 Dalton for loss of a water molecule. Both Q1 and Q3 scan over specified ranges of m/z values. In this mode, the detector records only those precursor ions that decompose, losing the specified neutral fragment. This type of MS experiments highly decreases chemical noise and is very helpful in identification of unknown SPLs.
Precursor Ion Scan (PI)
In a PI scan mode, the Q3 is set to pass specific m/z value, characteristic of a defined secondary ion. The Q1 scans across m/z range, recording only those primary ions which decompose to the specified product ion of interest. This highly specific scan mode eliminates or at least greatly reduces chemical noise and it constitutes a very useful identification tool since each class of SPLs yields at least one common product ion. Thus, setting Q3 to this specific daughter ion and scanning Q1 over the expected parent ion mass ranges, a spectrum of molecular species for an unknown biological sample may be identified.
Multiple Reaction Monitoring (MRM)
In a MRM experiment, the Q1 is set to pass specific precursor ion m/z and Q3 specific daughter ion m/z only.
This makes the MRM the most specific and sensitive MS/MS experiment allowing the analysis of even very minor components of a complex mixture with great precision and sensitivity. Such experiment practically eliminates chemical noise, thus makes it an ideal tool for quantitative analyses, particularly if coupled with HPLC physical separation. Multiple mass transitions, specific for particular compound, may be monitored sequentially; therefore, a large number of compounds may be analyzed together. Optimization of CID parameters for each compound of interest results in best sensitivity and specificity.
Sphingolipid Identification
Due to the complexity of sphingolipids, which usually constitute minor components of a crude lipid extracts, identification of individual molecular species is necessary before attempting any quantitative determination. This task can only be achieved with application of MS, particularly with precursor ion scan (PI) experiments. Although direct infusion full scan MS have been attempted,30,36 reliable results may be obtained only for negative mode, in which a limited number of SPLs, such as free fatty acids, are ionized. In full scan positive mode, high chemical background makes any identification virtually impossible.
Qualitative analysis of SPLs from crude extracts is best accomplished by analysis of their unique molecular decomposition products using a PI scan of common fragment ions, characteristic for the particular class of SPLs (Fig. 3).32,33,48 Readers are directed to the comprehensive studies on fragmentation patterns for mammalian and yeast SPLs, presented by Sullard18 and Shevchenko,54 respectively. Briefly, for mammalian SPLs, the m/z 264 and m/z 266 are the common fragment ions used for identification of Sph and its saturated counterpart, dhSph derivatives, respectively.
Considering the complexity of SPL composition, as well as the presence of many other lipid related compounds in biological material extracts, it is advisable to confirm initial identification, derived from PI scan, in order to avoid false identification. This may be accomplished by other, more compound-specific MS experiments, such as product ion scan of the newly identified molecular ion, or in a MRM experiment, with mass transition unique for the particular molecule e.g., single or double dehydration for Cers or NL of sugar moiety for GlcCers.
Ionization conditions and collision energy are optimized for individual molecular species to achieve maximum sensitivity and quantitative accuracy. SPLs composition has to be established for every new matrix.
Cer, CerlP and GlcCers’s molecular species (C18-SB) is established by the Precursor Ion scan, performed for the common Product Ion (m/z ) 264.2 and 266.1 for Sph and dhSph derivatives, respectively at the high collision energy (35-55 eV), operating in positive ionization mode (Fig. 3). A representative sample extract is infused directly into ESI source and it is then scanned for molecular ions of the potential SPLs. Further confirmation of identity is achieved through MRM analysis with "soft" fragmentation (15-30 eV). Running sample through the HPLC system also confirms a reasonable retention time. Only SPLs that satisfy identification criteria in both analyses should be considered truly present in the sample.
SM and dhSM molecular species (18C-SB). Identification of the SM and dhSM components is performed similarly, employing common Product Ion (m/z 183.9) at 40 eV collision energy (Fig. 3).
Note: It is important to optimize the ionization conditions for each class ofSPLs and collision energy for each individual molecular subspecies to be applied for quantitative MRM analysis.
HPLC-MS/MS Methodology
High Performance Liquid Chromatography (HPLC) is often employed for the separation ofintact lipid molecules using various detectors. SPLs lack chromophores that would have enabled direct specific spectrophotometric detection. Some attempts have been made with UV55-59 and evaporative light scattering (ELSD).60-62 Both detectors, however, lack specificity and impose additional limitations. With UV detection, it is very difficult to select a working mobile phase (MB) since underivatized SPLs absorb close to the 200-210 nm range, depending upon the degree of unsaturation of the FA moiety and most of the commonly used solvents strongly absorb in this region.59,63
In the ELSD detector, the HPLC column effluent is evaporated, leaving the solute components as fine droplets, which are illuminated by laser and the scattered light is measured. This is an indiscriminatory detection since any compound that does not evaporate may be detected.63
An alternative technique that overcomes most of the above problems and provides both compound specificity and quantitative sensitivity is the use of HPLC coupled with ESI/MS. It is one of most powerful technologies for analysis of intact polar lipid molecules. The physical separation power of HPLC into either various lipid subclasses and/or individual molecular species within the class, together with MS highly selective detection, makes possible simultaneous determination of either protonated or deprotonated molecules, providing also invaluable structural information.64
Both Normal (NP) and Reverse Phase (RP) HPLC have been employed and it is important to select a solvent system that renders chromatographic resolution and ESI/MS compatibility to achieve maximum sensitivity.
Recently, the most powerful technique applied for SPL molecular species is the HPLC-MS/MS instrumentation with the MRM scanning mode where each target analyte is uniquely identified by the Precursor-Product ion mass transition and the specific retention time.
Quantitation
Quantitation of SPLs in biological extracts has been a least developed segment of the HPLC/ MS/MS analysis due to very limited supply of commercially available individual standards. Only laboratories that have access to custom made synthetic standards41 were able to set up a reliable comprehensive quantitative protocols for various SPL classes. Recently, however, Avanti Polar Lipids Inc. (Alabaster, AL) and Matreya Inc. (Pleasant Gap, PA) significantly increased their offer of synthetic standards, so those major difficulties can be gradually overcome.
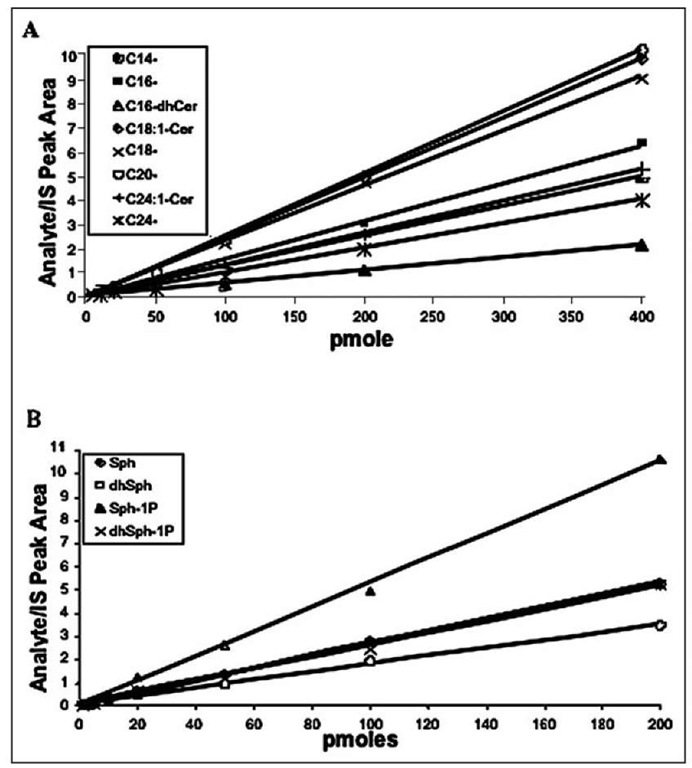
To achieve reliable quantitation of all molecular species, calibration curves should be generated for as many representative components of SPL as possible, due to diversified MS responses, as reflected by calibration curve slopes (Fig. 4).
Figure 4. Calibration curves of sphingoid bases and ceramides. The MS response varies for molecular species even within the particular SPL class, as indicated by the calibration curve slops; therefore, individual calibrations are generated for as many target analytes as possible. Linear instrument response (R2 value of .99) is obtained for the typical calibration ranges: 1.0-400.0 pmoles for SBs and SB 1Ps (lower panel), as well as for all ceramide species, Cn-Cers (upper panel).
Selection of Internal Standards (ISs)
Selection of a representative set of internal standards, which serve as a reference for both identification and quantitation, is critical for the analysis of a complex mixture of SPLs. Internal standards should be as close as possible to the target analytes, presenting similar MS fragmentation pattern as well as physicochemical properties reflected by similar solubility, extraction efficiency and mobile-stationary phase relationship during the HPLC separation. The best IS would be a compound which is chemically identical to target analyte labeled with a stable isotope, usually 2H or 13C. However, considering the large number of SPL molecular species, such approach is impractical; therefore, some compromise has to be applied. Often one IS per SPL class is used, mostly a sphingolipid with unnatural, usually lower, number of carbon atoms in the FA moiety.18,25,31-34,66,67
Bielawski et al.41 have introduced ISs for particular SPL classes synthesized from C17- SB as the closest "unnatural" sphingoid base to the natural C18-SB counterpart. This selection gives a confidence that physicochemical properties such as the elution order and mass fragmentation pattern accurately reflect natural SPLs, but are not present in the analyzed sample. Moreover, since they are introduced to the samples prior to extraction, incomplete extraction efficiencies are compensated for, rendering quantitation of the target SPLs more precise.
Quantitative Calibration
Generating a calibration mechanism for each target SPL in a class greatly improves the quality of the obtained quantitative results. However, due to the limited availability of authentic standards (see above), calibration for, as many as is practical, representative standards should be generated, so that calibration devised for the synthetic standard can be also used for few structurally closely related analytes.
Sometimes18,25,31-34,48,66,67 quantitation is performed using one IS as a single point calibration. This is not a very good practice since it assumes equal MS response to all molecular species in the class. Unfortunately, MS responses varies widely, depending on both structural features (number ofcarbon atoms, branching, unsaturation etc.) as well as mobile phase composition which changes over time, particularly when gradient elution is employed.
Based on the above considerations, we have adopted the following approach to SPL quantitative analysis.41 In this approach, quantitative analyses of SPLs are based on the eight-point calibration curves generated for each target analyte. The synthetic standards along with a set of ISs are spiked into an artificial matrix, then subjected to the identical extraction procedures as the test samples and then analyzed by the HPLC-MS/MS system operating in positive MRM mode, employing HPLC with gradient elution. Peaks for the target analytes and IS are recorded and processed using the instrument software system. Plotting the analyte/IS peak area ratios against analyte concentration generates the analyte-specific calibration curves. Any SPL for which no standard is available is quantitated using the calibration curve of the closest counterpart.
Data Handling
Results from the MS analysis represent the mass level of particular SPLs (in pmols) per total sample used for lipid extract preparation and quantitative analysis. In general, treatment with exogenous agents causes changes in SPL levels and compositions. For the final data presentation, MS results should be normalized to some stable parameters (which are considered not affected by that particular treatment). Total protein (mg), or phospholipid contents Pi (nmol) present in the Bligh & Dyer extract,27 which corresponds to the amount of the biological material used for MS analysis, can be used as the normalization parameters.29,68,69 Also normalization to the total cell number is used.70 Final results should be shown as changes in the relation to the control (% control). From our experience, data normalized to the protein or to the Pi (shown as % of the control) are not exactly the same. It is critical that once the user selects the normalization parameter, it carry it out consequently throughout the total study for consistency of the generated quantitative results.
Alternative Methodology
A variety of different techniques (mostly radio-labeling, HPLC analysis offluorescent analogs and enzymatic methods) in addition to MS methodology are used for SPLs measurement. Up to now, the enzymatic method employing diacylglycerol kinase and (32P) ATP has been the most commonly used procedure for total sphingolipids quantitation in the range of 25 pmols to 2 nmols.69 Cellular SBs are most often analyzed by the HPLC technique developed for their fluorescent derivatives.71 Cellular SB-1Ps are analyzed via their derivatization to (3H) C2-ceramide phosphate, by an enzymatic method (employs alkaline phosphatase), followed by action ofrecombinant sphingosine kinase and (32P ATP)) after TLC separation ofS1P from the cellular Sph, or by employing HPLC analysis ofOPA-derivatised S1P.72-76 These procedures require less expensive equipment than mass spectrometry but are not as informative. SM may be determined by several different approaches including TLC analysis, GC analysis of silylated derivatives and MS techniques.49,50,68,70 Total Cer and SM can be determined following hydrolysis and analysis of the liberated and derivatised SBs by means of HPLC71,74 and the liberated fatty acids by means of GC77 or GC/MS.78
Conclusion
This topic describes quantitative analysis of virtually all compounds involved in sphingolipid metabolism and turnover (signaling) such as sphingoid bases, sphingoid base-1-phosphates, lys-osphingolipids, ceramides, ceramide-1-phosphates, sphingomyelin and cerebrocides. The major emphasis was put on the most versatile LC/MS technology, that provides a wealth of structural information and specificity, essential in developing reliable analytical protocols, due to complexity of "sphingolipidome".
The LC/MS/MS methods have been successfully applied to a large number of different mammalian, and yeast cell lines, as well as various tissue samples, that typically contain many different sphingolipid subspecies, but constitute only a small fraction of crude lipid extracts.
So far there are not very good, mass spectrometry based analyses for some part of "sphingolipidome", namely complex gangliosides e.g GD3, due to lack of authentic, synthetic standards. Once this problem will overcome new analytical methodology will follow.