Abstract
Sphingolipids are important components of eukaryotic cells, many of which function as bioactive signaling molecules.1-3 As thoroughly discussed elsewhere in this volume, ceramide, central metabolite of the sphingolipid pathway, plays key roles in a variety of cellular responses. Since the discovery of the bioactive function of ceramide,4 a growing number of tools and techniques have been and still are being developed in order to better decipher the complexity and implications of ceramide-mediated signaling. With this topic it is our intention to provide new comers to the sphingolipid arena with a short overview of tools and techniques currently available for the study of sphingolipid metabolism, with the focus on ceramide.
Lipid Extraction
Isolation of ceramide from animal tissues and culture cells is achieved by extraction with organic solvents because of it’s nonpolar chemical structure. This is accomplished by using the methods described by Bligh and Dyer5 or Folch et al.6 Approximately 100% of the biological ceramide species are extracted into the organic phase under either method. When considering a more comprehensive analysis, it is important to consider the structural heterogeneity of the sphingolipid class members therefore specific lipid extraction methods might be required. For instance, simultaneous isolation of polar (such as sphingoid base phosphates or gangliosides) and nonpolar sphingolipids (such as ceramides) would be better achieved using a monophase extraction.
Identification and Quantification of Steady State Levels of Ceramide
Several methods have Deen developed to identify and quantify ceramide by mass spectrometry,7-10 thin layer chromatography (TLC),1U2high performance liquid chromatography (HPLC)13 and enzymatic assay using Escherichia coli diacylglycerol kinase (DGK).14,15 The term ceramide is often used for ^-acyl-sphingosine, however when investigators use a method that does not distinguish among backbone species (such as analyses by TLC or DGK assay), the term will likely include all N-acyl-sphingoid bases regardless of the back bone.
In the past, the most widely used techniques for the determination of steady state levels of ceramide in cells have been long-term radiolabeling and the diacylglycerol kinase assay.
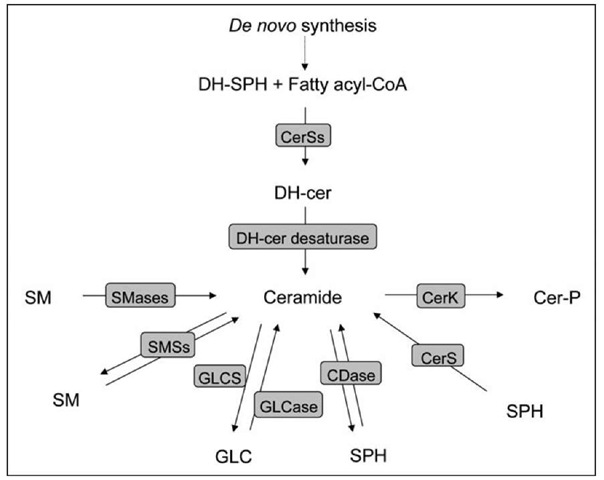
Radiolabeling of ceramide is based on the use of a radioactive precursor for ceramide synthesis. De novo ceramide synthesis begins with the condensation of L-serine and palmitoyl-CoA through the action of serine palmitoyltransferase to form 3-ketosphinganine, which is then reduced to sphinganine (dihydrosphingosine) (Fig. 1). This sphingoid base is then acylated by (dihydro)ceramide synthases (CerSI to 6) to form dihydroceramides with distinct fatty acyl moieties which are then desaturated by dihydroceramide desaturase to form the corresponding ceramides.
Figure 1. Schematic of ceramide metabolizing enzymes in mammalian cells. Represented are the reactions and the respective enzymes involved in the metabolism of ceramide. Some enzymes can catalyze a reaction both in forward and reverse mode (i.e. SMSs and CDase). DH-cer: dihydroceramide; DH-SPH, dihydrosphingosine; SM: sphingomyelin; GLC: glucosylceramide; SPH: sphingosine; Cer-P: ceramide phosphate; CerSs: ceramide synthases; SMases: sphingomyelinase; SMSs: sphingomyelin synthases; GLCS: glucosylceramide synthase; GLCase: glucosylceramidase; CDase: ceramidase; CerK: ceramide kinase.
Therefore radioactive palmitate, serine or the more proximal dihydrosphingosine have all been widely used as ceramide precursors. Two critical points must be considered when approaching this technique: the radioactive precursor used should be in trace amounts and the duration of the metabolic labeling should be optimized based on the chosen precursor and the experimental conditions (number of cells, volume of medium, type of medium etc.). The scientific rationale for this technique is based on the fact the radiolabeled precursor should enter the cellular sphingolipid pathway without perturbing it and it is used alongside its unlabeled counterpart present in the cells. Therefore the amount of radiolabeled ceramide present in the cell after steady-state labeling is representative of the total ceramide levels. Since the technique exploits the endogenous metabolic pathway, it is imperative that the amount of radioactive substrate added to the cells does not constitute a significant mass that could alter the flux through the pathway. Cells should be incubated with the radioactive precursors for the amount of time that allows the precursors to be steadily incorporated in the pathway until their conversion into ceramide (equilibrium between ceramide synthesis and ceramide degradation). This implies that a residual pool of not yet metabolized precursor should be still available to the cell and the ratio of radioactive ceramide to total phospholipids or total proteins remains constant with time. The amount of precursor to be added to the cells in order to obtain sufficient sensitivity (enough radioactivity associated with ceramide) greatly depends on the precursor’ specific activity. In general, 10-30 uCi of palmitate or dihydrosphingosine added to 2 million cells for 24 hours are enough to achieve all of the above.16-19 Different solvent systems may be used for separation of ceramide on TLC. We find that a mixture of ethyl acetate/iso-octane/acetic acid (9:5:2, v/v/v) is particularly suitable if ceramide is the lipid of interest. If one, in addition to ceramide, is also interested in more polar sphingolipids, such as sphingomyelin and gangliosides, then a mixture of chloroform/methanol/H2O (110: 40:6, v/v/v) would be our choice.
The DGK assay exploits the enzymatic promiscuity of the DGK enzyme. The DGK can in fact phosphorylate both DAG and ceramide because of the structural similarities between these two lipids. On the other hand, it needs to be pointed out that the Km for ceramide is almost five times greater than that for DAG and this becomes a critical factor to consider when setting up the proper in vitro conditions for reliable and quantitative ceramide measurements. Thus, after solubilization of total cellular lipid extracts in micelles, addition of recombinant DGK leads to phosphorylation of ceramide (and DAG). The addition of radioactive ATP allows the phosphorylated ceramide to be traced and measured. At completion of the reaction, lipids are extracted, separated by TLC and radioactivity associated with ceramide-P is determined. Conversion of radioactivity into mass is obtained by using a standard curve derived from phos-phorylation of known amounts of ceramide. The reliability of this assay depends on the fact that the reaction must be carried out to completion, which means that all the ceramide present in the original extract is converted into ceramide-P and that no factors in the lipid extracts may affect the DGK activity. In order for this to happen, the relative amounts of lipid extracts and the DGK used in the assay have to be carefully chosen. The user should work in the presence of great excess of DGK as compared to the amount of ceramide, so that the effect on the enzyme of potential inhibitors/activators can be dismissed and that the presence of DAG in the lipid extracts is not limiting. A thorough discussion on the use of DGK for ceramide quantification is presented by Dr. Bielawska and colleagues20 and accurate description of the assay is provided by Dr. Perry and colleagues.14
More recently much emphasis is given to the structural diversity of ceramide species. In fact, particular species of ceramide with distinct fatty acyl moieties were shown to selectively increase with cellular responses and generate specific ceramide signals.21,22 Therefore, the possibility of qualitatively and quantitatively isolate individual ceramide classes has significantly progressed the field. This task is accomplished using mass spectrometry methodology and the specific methods have been extensively described elsewhere.8,23-25
Analysis of Ceramide Metabolism
Radiolabeling of cells can also be used to specifically assay ceramide metabolism (either synthesis or break-down). For ceramide synthesis, the goal is to pulse the cells with trace amounts of a radioactive precursor and measure the levels of radioactive ceramide before the radiolabeling has reached steady state equilibrium. Generally few hours of incubation (up to 12 hours) will suffice. Since the pool ofceramide labeled in these conditions is smaller than the one achieved after steady state labeling (see previous section), the specific activity of the precursor is key in order to be able to detect enough radioactive signal associated with newly synthesized ceramide.
Ceramide break-down can be also measured by radiolabeling of cells. After labeling cells to steady state, cells are chased (incubated in absence of radioactive precursor), generally for 1 to 2 hours, to remove most of the still present free precursor. After a final wash, cells can be treated accordingly and subsequently collected and processed for lipid extraction as discussed in the previous section.
A combination of pulse labeling and chase experiments will provide important information on the nature of potential ceramide changes (either modulation of de novo synthesis or turnover).
With the advent of mass spectrometric analysis of sphingolipids, additional specific tools have been developed for detection of de novo ceramide synthesis. One of these is the use of C17-dihydrosphingosine. This particular precursor has a sphingoid backbone of seventeen carbons, which is not found in naturally occurring sphingolipids but it is recognized as substrate for ceramide synthesis. Therefore it can be used as proximal precursor for ceramide synthesis and its conversion into C17-dihydroceramide and more complex C17-sphingolipids can be detected by mass spectrometry.
The Use of Ceramide Analogues
Short-Chain Ceramides
The use of short-chain ceramides provided the first clue of the biologic functions that are affected/regulated by ceramide.26 Natural ceramides are very hydrophobic, resulting in very poor water-solubility. This physical property prevents their delivery to cells. One of the approaches to overcome this problem has been the development of synthetic water-soluble ceramide analogues that are mostly shortened in the fatty acid moiety (i.e., C2-ceramide, d-erythro-acetylsphingosine; C6-ceramide, d-erythro-hexanoylsphingosine).27 These short chain ceramides are more soluble and cell membrane-permeable, providing a viable tool to further characterize ceramide biology and identify target molecules using in vitro and in vivo systems.18,26,28 Exogenously added short-chain ceramides, besides being used for synthesis of more complex sphingolipids, undergo deacylation followed by recycling of the sphingosine backbone by reacylation with a long-chain fatty acid, generating long-chain ceramides.29 Therefore any target/biological effect identified by the use of these analogues may be in fact due to the accumulation of natural ceramide instead.
Fluorescent Ceramide Analogues
Fluorescent analogues such as Bodipy-ceramide, DMB-ceramide and NBD-ceramide are very useful in studies of ceramide transport and metabolism.30-32 In addition, short chain NBD-ceramides are also used as substrates for determining in vitro activities of ceramidases33 or sphingomyelin synthases.16
Pharmacological Tools
The discovery of small chemicals capable of inhibiting ceramide metabolizing enzymes in a potent and selective way could offer novel pharmacological tools for studying the biological role of distinct enzymes and new therapeutic reagents. A number of inhibitors for ceramide metabolizing enzymes have been developed or identified (Table 1).
Genetic Tools
A number of gene products contribute to the ceramide metabolism. Recent technologies have allowed selective gene silencing or knockout in mammals, providing powerful new tools for biological research and drug discovery.
RNA Interference
RNA interference (RNAI) represents a natural endogenous mechanism that cells utilize to regulate RNA expression.34,35 As a research tool, it is evident that RNA interference (RNAi) has revolutionized the biological science by allowing selective silencing of mRNA expression. Gene silencing can be induced by vector-based short hairpin RNA (shRNA) and synthetic small interfering RNA (siRNA) through a sequence-specific cleavage of perfectly complementary mRNA. In the field of sphingolipid research, RNAI has been used as a research tool for understanding metabolic and biological role for the ceramide metabolizing enzymes (Table 1).
Knockout Mice
The mouse is the foremost vertebrate experimental model because its genome can be precisely and variously engineered. Transgenic mice have allowed researchers to observe what happens to an entire organism during the progression of a disease and they have become models for studying human diseases and their treatments. A number of knockout mice of enzymes participating in ceramide metabolism have been generated (Table 1).
Table 1. Ceramide metabolizing enzymes and research tools in mammals
|
Gene Symbol |
Gene Name |
Inhibitors |
Availabiliy of si/shRNA for Human Genes |
KO Mice Availability |
Activity Assay |
|
ASAH1 |
Acid ceramidase |
B133 6 |
Available37 |
Lethal3,5 |
39 |
|
ASAH2 |
Neutral/alkaline ceramidase |
D-MAPP40 |
Available41 |
Survive42 |
33 |
|
ASAH3 |
Alkaline ceramidase 1 |
Available43 |
|||
|
ASAH3L |
Alkaline ceramidase 2 |
Available44 |
41 |
||
|
PHCA |
Alkaline phytoceramidase |
45 |
|||
|
CERK |
Ceramide kinase |
NVP-23146 |
Available47 |
Survive4,5 |
49 |
|
DEGS1 |
Dihydroceramide desaturase |
Cs-cyclopropenylceramide50 |
Available51 |
52 |
|
|
GBA1 |
Glucosylceramidase 1 |
Conduritol B epoxide53"55 Iminosugar54-56 |
Available57-5,5 |
Lethal after birth59 |
55 |
|
GBA2 |
Glucosylceramidase 2 |
Imino sugar60 |
Available57-5,5 |
Survive61 |
62 |
|
GBA3 |
Klotho-related protein |
Available63 |
63 |
||
|
LASS1/CerS1 |
Ceramide synthase 1 |
Fumonisin B164 |
Available21 |
65,66 |
|
|
LASS2/CerS2 |
Ceramide synthase 2 |
Available67 |
65,66 |
||
|
LASS3/CerS3 |
Ceramide synthase 3 |
65,66 |
|||
|
LASS4/CerS4 |
Ceramide synthase 4 |
65,66 |
|||
|
LASS5/CerS5 |
Ceramide synthase 5 |
Available21 |
65,66 |
||
|
LASS6/CerS6 |
Ceramide synthase 6 |
Available6,5 |
65,66 |
||
|
SGMS1 |
Sphingomyelin synthase 1 |
D6096,9 |
Available16‘7072 |
Survive* |
16,72 |
|
SGMS2 |
Sphingomyelin synthase 2 |
Available16‘7072 |
Survive73 |
16,72 |
|
|
SMPD1 |
Acid sphingomyelinase |
Desipramine54-74 (in vivo) |
Available37 |
Survive75 |
76,77 |
Table 1.
|
Gene Symbol |
Gene Name |
Inhibitors |
Availabiliy of si/shRNA for Human Genes |
KO Mice Availability |
Activity Assay |
|
SMPD2 |
Neutral sphingomyelinase 1 |
Available |
Survive7,3 |
79 |
|
|
SMPD3 |
Neutral sphingomyelinase 2 |
GW486930 |
Available32 |
Survive7,3‘33 |
84 |
|
Scyphostatin31 |
|||||
|
SMPD4 |
Neutral sphingomyelinase 3 |
Available7,3-35 |
|||
|
UGCG |
Glucosylceramide synthase |
PDMP36 |
Available3,3 |
Lethal39 |
90,91 |
|
AMP-DNM37 |
* Unpublished: Dr. Ken Watanabe (National Center for Geriatrics and Gerontology, Aichi, Japan).
Conclusion
Ceramide is at the heart of sphingolipid metabolism. As discussed, ceramide can be generated either de novo, or through the conversion of sphingosine (salvage pathway), or from the break-down of more complex sphingolipids such sphingomyelin (SM), glucosylceramide (Glucer) and ceramide phosphate (Cer-P). Therefore when studying ceramide metabolism, one needs to consider the number of interconnected reactions that contribute to the overall ceramide levels and the number of other metabolites that could themselves contribute to one or more cellular processes. Thus it is important to recognize that the more metabolic information are captured, the more complete and compelling the case will become.