Abstract
Gangliosides, characteristic complex lipids present in the external layer of plasma membranes, deeply influence the organization of the membrane as a whole and the function of specific membrane associated proteins due to lipid-lipid and lipid-protein lateral interaction. Here we discuss the basis for the membrane-organizing potential of gangliosides, examples of ganglioside-regulated membrane protein complexes and the mechanisms for the regulation of ganglioside membrane composition.
Introduction
Glycosphingolipids (GSL) are components of all animal cell membranes and, among these, gangliosides, sialic acid containing compounds, are abundant in the plasma membranes of neurons. Ceramide,1 the hydrophobic backbone of all sphingolipids, is constituted by a long chain amino alcohol, 2S,3R 2-amino-1,3-dihydroxy-octadec-4-ene (sphingosine), linked to a fatty acid by an amide bond. The hydrophilic head group of glycosphingolipids is an oligosaccharide chain, highly variable due to the sugar structure, content, sequence and connections. Sialic acid2 is the name that identifies all the derivatives of 5-amino-3,5-dideoxy-D-g/ycero-D-ga/acto-non-2–ulopyranosonic acid, or neuraminic acid. Three main sialic acids are known: the 5-N-acetyl-, the 5-N-acetyl-9-0-acetyl- and the 5-N-glycolyl-derivative. Healthy humans have only the first two,3-6 the 5-N-acetyl derivative being 85-90% of the total. The high Ka and the resulting negative charge confer them a strong amphiphilc character.
Due to the high heterogeneity of the both the hydrophibic and hydrophilic portion, glycosphingolipids are a very large family of natural compounds as shown in Table 1, were the main gangliosides from nervous system are reported.
Gangliosides (ganglioside nomenclature is in accordance with the IUPAC-IUBMB recommendations7) belong to external leaflet of the plasma membrane8 and are highly enriched in the pre and postsynaptic membranes of the synaptic terminals. With their hydrophilic oligosaccharide chains, they face the extracellular environment, providing ideal sites of interaction with extracellular molecules, including toxins, matrix components, adhesion molecules and specific receptors and enzymes on the surface of adjacent cells (trans interactions). Moreover, they have proven to be able to laterally interact with proteins belonging to the same membrane, modulating their biological functions (cis interactions).9-29
In the last 10 years it has emerged the concept that GSL possess a high potential for the creation of lateral order in biological membranes, contributing to the creation and/or stabilization of membrane macro- and microdomains.
Table 1. Structures of the main Neu5Ac containing gangliosides in vertebrates
|
Series |
|
Ganglioside |
||
|
Series |
Abbreviation |
Series Structure |
Abbreviation |
Ganglioside Structure |
|
Gal |
GalCer |
Cal-(1 -1 )-Cer |
GM4 |
Neu5AcGalCer |
|
Lac |
LacCer |
b-Cal-(1 -4)-b-Clc-(1 -1 )-Cer |
GM3 |
H3Neu5AcLacCer |
|
GD3 |
I l3(Neu5Ac)2 LacCer |
|||
|
0-acetyl-GD3 |
I !3[Neu5,9Ac2-(2-8)-Neu5Ac] LacCer |
|||
|
Ganglio-3 |
Gg3Cer |
b-GalNAc-(1 -4)-b-Gal-(1 -4)-b-Glc-(1 -1)-Cer |
GM2 |
H3Neu5AcGg3Cer |
|
GD2 |
H3(Neu5Ac)2Cg3Cer |
|||
|
Ganglio-4 |
Gg4Cer |
b-Gal-(1 -3)-b-GalNAc-(1 -4)-b-Gal-(1 -4)-b-Glc-(1 - |
GM1 |
H3Neu5AcCg4Cer |
|
1 )-Cer |
||||
|
GM1 b |
IV3Neu5AcCg4Cer |
|||
|
GD1a |
IV3Neu5Acll3Neu5AcCg4Cer |
|||
|
GD1b |
H3(Neu5Ac)2Gg4Cer |
|||
|
GD1b-lactone |
H3[Neu5Ac-(2-8,1-9)-Neu5Ac]Cg4Cer |
|||
|
GT1 a |
IV3(Neu5Ac)2ll3Neu5AcCg4Cer |
|||
|
GT1 b |
IV3Neu5Acll3(Neu5Ac)2Cg4Cer |
|||
|
O-Acetyl-GTI b |
IV3Neu5Acll3[Neu5,9Ac2-(2-8)-Neu5Ac]Cg4Cer |
|||
|
GT1 c |
H3(Neu5Ac)3Gg4Cer |
|||
|
GQ1b |
IV3(Neu5Ac)2ll3(Neu5Ac)2Cg4Cer |
|||
|
GQ1 c |
IV3Neu5Acll3(Neu5Ac)3Cg4Cer |
|||
|
GP1 c |
IV3(Neu5Ac)2ll3(Neu5Ac)3Cg4Cer |
|||
|
Fuc-GM1 |
IV2aFucll3Neu5AcCg4Cer |
Table 1.
|
Series |
Series Abbreviation |
Series Structure |
Ganglioside Abbreviation |
Ganglioside Structure |
|
Fuc-GD1b |
IV2aFucll3Neu5Ac2Gg4Cer |
|||
|
GD1a |
IV3Neu5Aclll6Neu5AcCg4Cer |
|||
|
Chol-1 a-a |
IV3Neu5Aclll6Neu5Acll3Neu5AcCg4Cer |
|||
|
Chol-1 p |
MI6Neu5Acll3(Neu5Ac)2Gg4Cer |
|||
|
GT1a |
IV3Neu5Aclll6(Neu5Ac)2Cg4Cer |
|||
|
GQ1a |
IV3(Neu5Ac)2lll6(Neu5Ac)2Cg4Cer |
|||
|
Chol-1 a-b |
IV3Neu5Aclll6Neu5Acll3(Neu5Ac)2Cg4Cer |
|||
|
Ganglio-5 |
Gg5Cer |
b-GalNAc-(1 -4)-b-Gal-(1 -3)-b-GalNAc-(1 -4)-b-Gal-(1 -4)-b-Glc-(1 -1 )-Cer |
GalNAc-CM1 CalNAc-GD1a |
M3Neu5AcCg5Cer IV3Neu5Acll3Neu5AcGg5Cer |
|
Neolacto-4 |
nLc4Cer |
b-Gal-(1 -4)-b-GlcNAc-(1 -3)- b-Gal-(1 -4)-b-Glc-(1 -1 )-Cer |
3′-LM1 |
IV3nLc4Cer |
Lipid membrane microdomains, or lipid rafts, areas in the membrane characterized by a lateral organization dictated by the properties of their lipid components, have been involved in several biological events.31-39 The coexistence of lipids in different physical phases within the same model membrane was probably the first evidence leading to the concept of lipid rafts. Lipid bilayers at physiological temperature usually exist in a liquid-disordered (ld) phase characterized by high fluidity, in which the lipid acyl chains are disordered and highly mobile. Lowering the temperature below the melting point freezes the lipid acyl chains in an ordered gel phase with very limited freedom of movement. Membrane lipids can also exist in a third physical phase, the liquid-ordered (lo) phase, in which the acyl chains of lipids are extended and ordered, as in the gel phase, but have higher lateral mobility in the bilayer. The lo phase is stabilized by the presence of cholesterol, that fill the hydrophobic gaps between the phospholipid or glycolipid acyl chains.40,41 Lipid rafts and GSL-rich microdomains are more ordered than the ld phase, being in this regard similar to a lo or a metastable gel phase.
Lateral phase separation of complex lipids in phospholipid bilayers can be observed in binary mixtures of diacyl lecithins differing in chain length and/or saturation42-44 and in ternary mixtures of palmitoyloleyl PC, dioleyl PC and cholesterol. However, GSL strongly differ from glycerolipids for their molecular structure and conformational properties, thus leading to a strong tendency to segregate within phospholipid bilayers (reviewed in ref. 41). Gangliosides display the ability to associate with a pre-existing ordered domain or to segregate in their own domains, that can be distinct from the cholesterol-enriched phase. The segregation of one ganglioside component with respect to the other has been observed in mixed micelles of two gangliosides with the same acyl chain but different hydrophilic headgroups.45,46 Lateral separation of gangliosides also occurs in one-component as well as in two-component, two-phase phosphatidylcholine bilayers and in phospholipid bilayers in the presence of cholesterol and/or sphingomyelin.47-55 The formation of separate GM1-enriched and cholesterol-enriched liquid-ordered phases was observed56 in ternary sphingomyelin/GMl/cholesterol vesicles and in lipid monolayers.57
Studies conducted on model membranes led to the following statements. (1) The presence of an amide nitrogen, of a carbonyl oxygen and of a hydroxyl group at the water/lipid interface in the common hydrophobic ceramide backbone confers to sphingolipids to act both as donors and acceptors for the formation of hydrogen bonds58; this feature is unique for sphingolipids among complex membrane lipids and the formation of a hydrogen bond network at the water/ lipid interface strongly stabilizes the segregation of a rigid segregated phase enriched in sphingolipids. (2) In the case of GSL, the presence of the bulky oligosaccharide hydrophilic headgroup termodinamically favours their segregation within a biological membrane. Phase separation with clustering of GSL in a phospholipid bilayer is a spontaneous process driven by the minimization of the interfacial free energy. The interfacial area increases with the size of the oligosaccharide chain, with a corresponding more pronounced segregation.59-71 These predictions based on the geometrical parameters of the GSL molecules are experimentally confirmed by the observation that phase separation is present in mixed micelles of two different gangliosides45,46 with the same fatty acid composition and that the extent of ganglioside phase separation in glycerophospholipid bilayers depend on the surface area occupied by the GSL oligosaccharide chain, that is usually increasing with the number of sugar residues.50-52 ( 3) Membrane complex lipids, in particular glycerophospholipids, are highly heterogeneous in their fatty acid composition. The hydrophobic portion of complex lipids is very heterogeneous, but in sphingomyelin and gangliosides (at least in the nervous system), saturated acyl chains like palmitic and stearic acid are the main components72 (Table 2). The presence of saturated acyl chains (that can be tightly packed with a high degree of order in the hydrophobic core of a bilayer) favours the phase separation of a rigid, liquid-ordered phase. In the case of GSL, for GM1 it has been shown that distribution in the fluid phase of a phospholipid bilayer54 is inversely correlated with the acyl chain length and directly correlated with the degree of unsaturation.
These data clearly demonstrate that sphingolipids form laterally separated phases characterized by reduced fluidity and hydrocarbon chain mobility. An hypothetical model for sphingolipid segregation in cell membranes is presented in Figure 1.
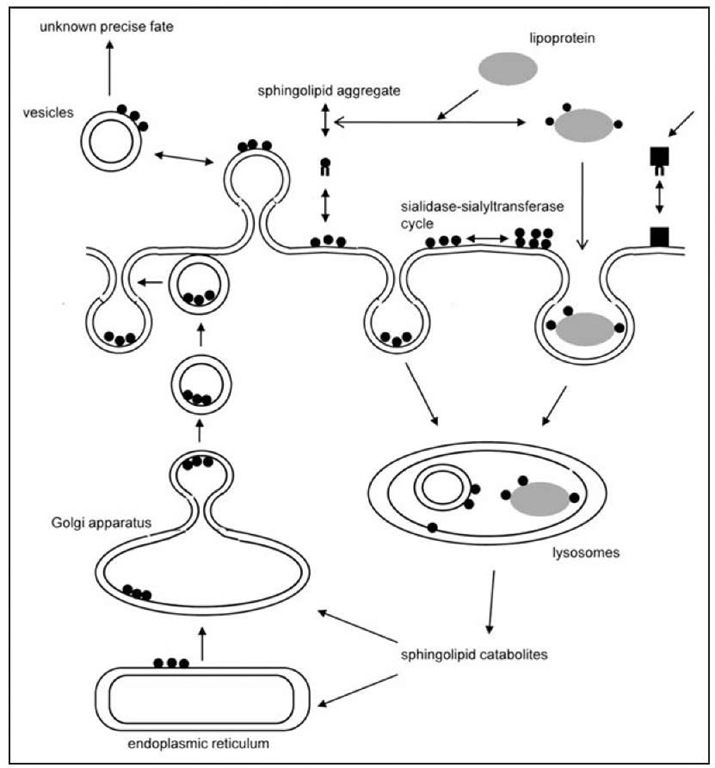
Figure 1. The scheme shows the different metabolic pathways that determine the membrane ganglioside content and pattern. Neo-biosynthesis occurs through the ER and Golgi apparatus the, but shedding of vesicles and monomers, together with a sialidase-sialyltransferase cycle participate to determine the finale ganglioside content and composition of the membrane.
Segregation of membrane sphingolipids is responsible for the creation of less fluid membrane regions, where membrane-associated proteins can be confined, favouring lateral interactions between sphingolipids and/or proteins that are segregated in the same lipid domain or preventing interactions between proteins that are associated with different domains.
Segregation of Membrane Lipids and Detergent-Resistant Membrane Domains
The interest for lipid membrane domains became very strong when many proteins deputed to cell signaling were found to be preferentially associated with an environment of lipids highly enriched in sphingolipids and cholesterol. The possible role of lipid membrane domains in the transport of GPI-anchored proteins from the Golgi apparatus to the apical plasma membrane of polarized cells originated the now widely used term "lipid rafts".73
Originally, the existence of a membrane fraction characterized by a peculiar lipid composition leading to a liquid-ordered or highly organized phase was operationally defined on the basis of the insolubility in aqueous nonionic detergents.74 Most components of the cell membrane are solu-bilized by detergents.75 In contrast, many cellular components are insoluble in nonionic (Triton X-100) under certain experimental conditions. After detergent treatment, the detergent insoluble membrane domain can be separated from the rest of the cell thanks to its relative light density (due to its richness in lipids),74 using continuous or discontinuous density gradients. Low-density, detergent-insoluble fractions were isolated from a wide variety of cultured cells, including almost all mammalian cell types76-91 and tissues92-98 investigated so far, yeasts99 and protozoans.
Table 2. Ratio between the components of sphingolipid-cholesterol enriched membrane fraction in rat cerebellar granule cells differentiated in culture
|
|
Molar Ratio, Gangliosides as 1 |
Molar Ratio,% |
Asymmetry, Layer |
|
Proteins |
0.04 |
0.26 |
Both, largely inner |
|
Ceramide |
0.20 |
1.32 |
Outer |
|
Sphingomyelin |
1.40 |
9.20 |
Outer |
|
Gangliosides |
1.00 |
6.63 |
Outer |
|
Phosphatidylcholine |
7.00 |
46.45 |
Outer |
|
Plasmalophosphatidylcholine |
0.01 |
0.07 |
Outer? |
|
Phosphatidylserine |
0.40 |
2.65 |
Inner |
|
Phosphatidylethanolamine |
0.70 |
4.64 |
Largely Inner |
|
Plasmalophosphatidylethanolamine |
0.10 |
0.66 |
Inner? |
|
Phosphatidylinoisitol |
0.10 |
0.66 |
Largely Inner |
|
Phosphatidylinoisitolmonophosphate |
0.01 |
0.07 |
Inner |
|
Phosphatidylinoisitoldiphosphate |
0.01 |
0.07 |
Inner |
|
Cholesterol |
4.10 |
27.21 |
Both, largely inner |
In following time, a wide range of different detergents95,96,101,102 was used and several "detergent-free" procedures for the separation of low-density membrane fractions corresponding to lipid membrane domains were developed.103,104 When comparatively analyzed, low-density membrane fractions obtained after cell lysis under the dramatically different experimental conditions described above, are very similar but not identical.77,90,91,105-116 Low-density membrane fractions always contain a highly resistant supramolecular structure possible corresponding to the native core of lipid membrane domains suggesting that the low-density membrane fraction composition correspond to that of physiological lipid membrane domains and that it is not determined by a random rearrangement of cell components induced by the experimental conditions used. Nevertheless, it cannot be excluded that the differences observed by some Authors might be simply due to contingent situations, since the standardization of the experimental procedures is sometime difficult and the overall composition of DRM fractions or the association of specific molecules with it seem to be affected by even tiny modifications of several conditions, including agents used for membrane disruption,77,93,95,98,101,104,110 mechanical procedures used to obtain or aid membrane solubilization,110 temperature74,101,116,117 and ratio between detergent and biological material.98,118 However, several studies indicate that at least in some cases the differences observed in the composition of low density DRM fractions isolated by different methods might reflect the existence of different levels of order within lipid membrane domains and/or of biochemically distinct lipid membrane domains within the plasma membrane of the same cell. This is particularly clear if the results obtained by the use of different detergents are compared.74,101,119-122 Thus, differential detergent solubilization might prove to be a powerful tool to study different lipid membrane domain subpopulations.
The existence of lipid membrane domains in natural cell membranes was suggested by the observation that glycosphingolipids at the cell surface form clusters, which have been visualized by immuno-electron microscopy using antiglycosphingolipid antibodies.79,123-126 Several approaches, relying on more advanced technologies including single-particle tracking or single fluorophore tracking microscopy,127-132 fluorescence recovery after photobleaching,133 fluorescence resonance energy transfer134,135 and atom force microscopy136 are now available allowing the detection and the study of lipid membrane domains in intact cell membranes.137,138 However, these techniques are very heterogeneous and and data obtained with different approaches are sometime conflicting. As example, there is no agreement on their average size that ranges from 26 nm to about 2.
Lipid Membrane Domain Functions
Gangliosides and Lipid Membrane Domains in the Nervous System
Neuronal and glial lipid membrane domains, prepared from cultures of neural cells or nervous tissues118,145,146 are rich in gangliosides, sphingomyelin, cholesterol and proteins involved in mechanisms of signal transduction that are relevant for neuronal functions.90,91,94,102,106,108,117,147-168 Lipid membrane domains in the nervous system cells has been involved in neurotrophic factor signaling,149-152 cell adhesion and migration,150,158,162 axon guidance, synaptic transmission,150,157 neuron-glia interactions163,164 and myelin genesis.165 The involvement of lipid membrane domains in neuronal and glial signal transduction includes several different ways: 1) receptors and effector proteins permanently resident in lipid membrane domains can be activated, giving rise to signal propagation that involves other components intrinsically present in the lipid membrane domain. Examples are neurotrophin receptors of the trk family, EGFR, PDGFR, p75NTR, GFRa149-152 and the neural cell adhesion molecule TAG-1.106,166,169 Src family tyrosine kinases are among the effector signaling proteins that are most commonly engaged in these cases; 2) the activation of membrane receptors is followed by the recruitment to lipid membrane domains of receptors themselves or effector signaling proteins that are not located in lipid membrane domains under basal conditions, or, the activation of receptors that are associated with lipid membrane domains under resting conditions determines their translocation outside lipid membrane domains. Examples of the former are the receptor tyrosine kinase c-Ret, recruited into lipid membrane domains by its GPI-anchored coreceptor GFRa149,150,152 and the neuronal adhesion receptor NCAM, recruited into lipid membrane domains by cis- or trans- interaction with its membrane bound, GPI-anchored ligand, prion protein.162
Both modes implies changes in the reciprocal interactions of lipid membrane domain components. Changes in the lipid and/or protein composition of lipid membrane domains and in the interactions of the lipid (in particular glycosphingolipid) components with specific proteins of functional relevance could thus be very relevant during the process of neuronal adhesion, survival, migration, differentiation and senescence.
Properties of lipid membrane domains from rat cerebellar granule cells at different stages of development in culture are available.72 The surface occupied by these structures increased during development, with the maximum ganglioside density in fully differentiated neurons. On the other hand, a high content of ceramide was found in the domains of aging neurons. The sphingolipid/ glycerophospholipid molar ratio was more than doubled during the initial stage of development, corresponding to axonal sprouting and neurite extension, whereas the cholesterol/glycerophos-pholipid molar ratio gradually decreased during in vitro differentiation. Phosphorylated phos-phoinositides were very scant in the domains of undifferentiated cells and dramatically increased during differentiation and aging.
Src family protein tyrosine kinases, (c-Src, Lyn and Fyn) known to participate to the process of neuronal differentiation, were associated with the lipid membrane domains in a way specific for the type of kinase and for the developmental stage of the cell.72 Within the lipid membrane domains, ganglioside GM3 has been found closely associated with c-Src and Csk in neuroblastoma Neuro2a cells90 and GD3 associated with Src-family kinase Lyn and the neural cell adhesion molecule TAG-1 in rat brain94,169 and cerebellar granule cells. In these cells, a complex lipid environment characterized by the presence of many ganglioside species and other membrane lipids (mainly cholesterol and dipalmitoylphosphatidylcholine) is essential for the interaction with the domain of c-Src, Lyn, Fyn, TAG-1 and prion protein.72,102,117,148 The presence of Src family nonreceptor tyrosine kinases in lipid membrane domains of neurons is particularly interesting, because many facts indicate that c-Src and other kinases of this family are important in the process of neuronal differentiation and in neuronal function.170-177 As mentioned above, in neuroblastoma Neuro2a cells, c-Src and Csk, are associated with GM3 ganglioside within lipid membrane domain and neuritogenic concentration of gangliosides are able to induce c-Src activation followed by mitogen-activated protein kinases activation.90 In these cells, anti-GM3 antibody is also able to induce differentiation.174 In rat cerebellum and cerebellar neurons, GD3 ganglioside is associated with Lyn and the neural cell adhesion molecule TAG-1 and antibody-mediated cross-linking of TAG-1 or GD3 induce Lyn activation.94,106,169 Glycosphingolipids were essential for TAG-1-dependent signaling via Lyn and for the maintenance of the differentiated neuronal phenotype, since incubation of cerebellar neurons with the glycosphingolipid-degrading enzyme endoglycoceramidase in the presence of its activator protein reduced the levels of cell surface glycosphingolipids, caused the redistribution of TAG-1 from nondomain membranes to the lipid membrane domain fraction, abolished TAG-mediated Lyn activation and consequent phosphorylation of p80 and induced neurite retraction.169
A possible role of lipid membrane domains in the pathogenesis of spontaneous and transmissible neurodegenerative diseases was recently highlighted by the discovery that a number of molecules causally connected to such diseases are associated with these domains. The most prominent examples are represented by the amyloid precursor protein (APP) in Alzheimer’s disease and by the prion protein. In both cases, the generation of the aberrant forms of these proteins, which are responsible for the onset of the disease, seems to be localized in the lipid membrane domains and/ or dependent from the structure of the domain itself.178,179
The Glycosynapse
Altered GM3 ganglioside expression plays a multiple role in the control of tumor cell motility, invasiveness and survival. Adhesion of B16 melanoma cells (expressing high levels of GM3) to endothelial cells, that express LacCer and Gg3, is mediated by GM3-LacCer or GM3-Gg3 interaction and leads to enhanced B16 cell motility and thereby initiates metastasis.105,180 GM3 is highly expressed in noninvasive, superficial bladder tumors compared with invasive bladder tumors, where the activities of glycosyltransferases responsible for GM3 synthesis were consistently upregulated.181,182 Enhanced GM3 expression achieved by pharmacological treatment with brefeldin A,181,183 or the exogenous administration of GM3182 suppressed the tumorigenic activity and/or the invasive potential of human colonic and bladder tumor cell lines and the stable over expression of GM3 synthase in a mouse bladder carcinoma cell line reduced cell proliferation, motility and invasion with concomitant increase in the number of apoptotic cells.184 High expression levels of GM3 with concomitant expression of the tetraspanin CD9 in colorectal185,186 and bladder181 cancer cell lines inhibited Matrigel and laminin-5-dependent cell motility.
At the molecular level, GM3 control on the properties of tumor cells requires a complex supermolecular membrane organization that defines highly specialized detergent-insoluble lipid membrane domains. The term "glycosynapse" has been proposed by S. Hakomori187,188 to generally describe a membrane microdomain involved in carbohydrate-dependent adhesion. Carbohydrate-dependent adhesion in glycosynapse, occurring through GSL-GSL interactions or through GSL-dependent modulation of adhesion protein receptors (such as integrins) leads to signal transduction events reflecting in deep changes in the motility and invasiveness of tumor cells. In the case of GM3-dependent adhesion of melanoma cells, it has been shown that GM3 is closely associated with c-Src, Rho and Ras within glycosphingolipid-enriched membrane domains and binding with Gg3 or anti-GM3 antibody stimulates focal adhesion kinase phosphorylation and c-Src activity.105 This molecular assembly defines a classically Triton X-100 insoluble G SL-enriched microdomain ("glycosynapse 1"), that can be isolated and separated from a caveolin-containing low-density membrane fraction in B16 cells.189 A similar association between a sialoglycolipid and c-Src and other related signaling molecules was observed for GM3 also in neuroblastoma cell,90 for disialylgalactosylgloboside in renal carcinoma cells190 and for monosialyl-Gb5 in breast carcinoma cells.191 Tetraspanin CD9 and integrin a3 or a5 also are colocalized within a distinct low-density, Brij 98-insoluble glycolipid-enriched domain ("glycosynapse 3"). The presence ofGM3 positivelymodulated CD9/integrin association. In fact, association between CD9 and integrin in the Chinese hamster ovary mutant cell line ldlD14 (deficient in UDP-Gal-4-epimerase) has been shown by co-immunoprecipitation experiments when cells were grown in the presence of galactose, allowing GM3 synthesis, or supplementing cells with exogenous GM3 Colocalization ofCD9, a3 and GM3 in cells was observed in intact ldlD14 cells in the presence but not in the absence of galactose.192 The formation of a3/CD9/GM3 complexes strongly inhibited the laminin-5-dependent motility in ldlD14 cells. On the other hand, it has been shown that CD9/GM3 complexes are essential for the regulation of integrin-mediated cell adhesion and signal transduction in oncogenic transformation, suggesting a crucial role for GM3 complexed with CD9 and integrin a3P1 or a5P1 in the control of tumor cell motility and invasiveness. v-Jun-transformed mouse and chicken embryo fibroblasts were characterized by lower GM3 levels and down—regulated GM3 synthase mRNA levels respect to the nontransformed counterparts.193 Reversion of oncogenic phenotype of v-Jun-transformed cells to normal could be achieved by enhanced GM3 synthesis through its gene transfection. When v-Jun-transformed cells were transfected with GM3 synthase expression plasmid, leading to increased GM3 synthase activity and GM3 cellular levels, their ability anchorage-independent growth in agar was strongly reduced. During phenotypic reversion induced by GM3 synthase transfection, the association of CD9/a5P1 complex (shown by co-immunoprecipitation and confocal microscopy experiments) was increased. Remarkably, the N-glycosylated form of P1 integrin was preferentially associated with the complex in GM3 synthase gene transfectants.193 GM3 levels were 4-5 times higher in the noninvasive KK47 cell line (originated from superficial human bladder cancer) than in the invasive YTS1 human bladder cancer cell line. Knock down of CD9 or pharmacologically achieved GM3 depletion in KK47 cells induces the phenotypic conversion to invasive variants. On the other hand, exogenous GM3 addition induces the phenotypic reversion of the highly invasive and metastatic cell lines YTS1 to low motility variants. The changes in cell motility were strictly correlated with the association of CD9 with a3 integrin. This interaction was higher in nonivasive than in highly invasive cells and was modulated by the cellular levels of GM3: CD9/a3 integrin association was reduced by GM3 depletion in KK47 and conversely enhanced by exogenous GM3 addition in YTS1 cells. GM3 levels in glycosynapse controls not only CD9/ a3 integrin association, but also the activation state of c-Src. c-Src is present in higher amount in the glycosynapse fraction in YTS1 cells and it is activated in cells with low GM3 levels and high invasive potential (YTS1 or GM3-depleted KK47). On the other hand, exogenous addition of GM3 to YST1 cells caused Csk traslocation to the detergent-insoluble fraction and consequent inactivation of c-Src, influencing cell motility.194
GM3 and EGF Receptor
The function of growth factor receptors can be modulated by gangliosides13 and lon time ago epidermal growth factor receptor (EGFR) was identified as the target of the inhibitory action of GM3.195 GM3 inhibited EGFR autophosphorylation without competing with EGF for receptor binding13,196,197 and without affecting receptor dimerization.198 The sialyllactose oligosaccharide is essential for ganglioside-receptor interaction and that the substitution with any other sugar negatively affects the binding.199 However, the molecular basis of this interaction has been only recently fully elucidated, emphasizing the importance of side-by-side carbohydrate-carbohydrate interactions between GM3 oligosaccharide and a N-linked glycan bearing multiple GlcNAc terminal residues on the receptor.200,201 GM3/EGFR interaction is facilitated by the enrichment of EGFR in classical ganglioside-enriched, cholesterol-sensitive, Triton X-100 insoluble membrane domains.202,203 However, other GSL- and lipid raft-dependent factors can affect EGFR function. Caveolae and caveolin-1 are involved in the modulation of EGFR signaling204,205 and EGFR is localized within a caveolin-rich fraction in A431 cells. However, EGFR-containing membrane fragments can be separated from caveolae.110,206 In a keratinocyte-derived cell line, GM3 over expression induced a shift of caveolin-1 to EGFR-rich membrane regions, allowing its functional interaction with the EGFR receptor, that caused inhibition of EGFR tyrosine phosphorylation and dimerization.207 Thus, GM3 influences EGFR signaling by a second distinct molecular mechanism modulating EGFR/caveolin-1 association. Moreover, GM3 negatively regulates as well the ligand-independent cross-talk of EGFR with integrin receptor signaling, disrupting when accumulated in cultured cells, the interaction of integrin P1 subunit with EGFR.208
GM3, Caveolae and the Regulation of Insulin Receptor and PDGF Receptor
Insulin receptors (IR) are present in detergent-resistant membranes from normal adipocytes209 and localized in caveolae in intact cells,210 where the P-subunit of IR interacts with caveolin-1 through a binding motif recognizing the scaffold domain of caveolin-1.211 IR can form distinct complexes with caveolin-1 and GM3 within lipid membrane domains.212 The interaction between GM3 and IR is direct and specific and was abolished in IR mutants where the lysine residue at 944 was replaced with arginine, valine, serine, or glutamine, suggesting that an electrostatic interaction between the negatively charged sialyllactose chain of GM3 and the positively charged amino group of lysine 944, located in close proximity to the transmembrane domain sequence of IR, is essential for the formation of the GM3/IR complex. In 3T3-L1 adipocytes, the induction of insulin resistance by treatment with TNFa was accompanied by the upregulation of GM3 synthase, leading to an increase ofcellular GM3,210,213 that accumulated in detergent-resistant membranes. In insulin resistance, the association of IR with GM3 was increased, while its association with caveolin-1 was decreased, indicating that the excess amount of GM3 in lipid membrane domains leads to the displacement of IR from the complex with caveolin-1, thus suggesting that the regulation of IR/ caveolin-1 by GM3 could be responsible for the changes in insulin response in adipocytes.212
A similar regulatory mechanism has been recently observed for PDGFR.214 Overexpression of the N-terminal domain of PAG, caused the accumulation of GM1 at the cell surface, with the consequent displacement of PDGFR from caveolin-rich fractions and caveolae, without altering the caveolar distribution of caveolin-1. The same redistribution of PD GFR has been observed after incubation of cells with exogenous GM1. Increased GM1 cellular levels lead to the displacement of another growth factor receptor, PDGF, from caveolae,214 negatively regulating Src mitogenic signaling. However, in this case it is not known whether the formation of a PDGFR-GM1 complex is required for its uncoupling from caveolae. Since caveolin-1 can direct bind sphingolipids, including GM1, in this case it cannot be excluded that GM1 forms a complex with caveolin-1, or that an enrichment in GM1 inside the caveola induces a deep reorganization ofcaveolar membrane, thus excluding PDGFR from caveolae.
The Regulation of Glycosphingolipid Composition of the Plasma Membranes
Changes in the glycosphingolipid composition of the plasma membrane would predictably lead to very important biological consequences, thus all mechanisms possibly contributing to these changes have a high functional significance. Changes in the activities of enzymes of the biosynthetic pathway residing in Golgi apparatus have been associated with the changes in GSL expression that are associated with neoplastic transformation or neuronal differentiation. However, both catabolic and biosynthetic enzymes for glycosphingolipids have been found associated with the plasma membranes, thus local mechanisms for the regulation of the gly-cosphingolipid composition of plasma membranes or restricted plasma membrane areas could be very important. The membrane-bound sialidase Neu3 has been identified and cloned215-217 and its trans activity in modifying the structure of gangliosides of adjacent cells has been proven.218-220 Moreover, the presence of sialyltransferase activities at the cell surface has been also reported.221-225 Thus, sialylation/desialylation cycles might be very important mechanisms responsible for rapid and possibly transient changes of the plasma membrane ganglioside content and pattern. The presence of other active glycosylhydrolases, P-glucosidase, P-galactosidase and P-hexosaminidase,220,226 in the plasma membrane has been demonstrated, implying that local hydrolysis of glycosphingolipids at the cell surface might represent a general mechanism for the control of glycosphingolipid composition. Moreover, gangliosides, as well as the other sphingolipids, can be released from the cell surface in different forms, including shedding vesicles,227-229 whose controlled release from specific sphingo(glyco)lipid-enriched membrane areas, could represent a further way to modify the lipid membrane domain composition and organization. The mechanisms responsible for he control of plasma membrane glycosphingolipid composition are depicted in Figure 2.
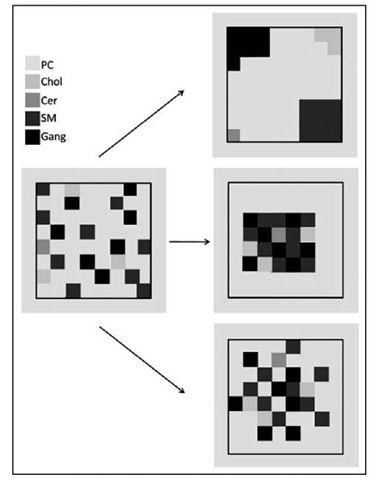
Figure 2. The figure shows the possible distribution of lipids belonging to the outer layer of a lipid raft according to the pattern reported in Table 2. According to the well known concept that the outer layer requires a larger surface with respect to the inner layer, that sphingolipids and phosphatidylcholine are components of the outer layer and all together cover about 60% of the total lipid raft content, the larger portion of cholesterol must be located in the inner layer. This is in agreement with published data.230231 Protein are not shown, but the larger portion should be associated to the inner layer, the main proteins recognised in lipid rafts, at least from a quantitative point of view, being those of cytoskeleton.166232233 In the panels only the elements inserted in the square belong to the lipid rafts. The left panel shows a random distribution of phosphatidylcholine, sphingomyelin, ceramide, gangliosides and a few molecules of cholesterol. These molecule must be organised as separated phases. We present three different possibility in the right portion of figure. The upper panel shows five different area each one covered by the same species. The central panel shows all the sphingolipids in a single phase. The lower panel shows a phase where some phosphatidylcholine is distributed within sphingolipids. This lipid distribution has been experimentally proved.52 Specific sphingolipid-protein interactions can modify this phase separation promoting subdomain organization.
Conclusion
In this topic, we describe the properties of gangliosides that underlie their roles as organizers of membrane organization and function. The potential for gangliosides in determining the properties of specific membrane areas characterized by a selective enrichment in these lipids as well as their ability to engage specific lateral interactions with membrane proteins have been discussed with the aid of examples covering the recent literature. In addition, we discussed the possibility that several metabolic pathways could affect the local ganglioside composition of specific membrane districts, thus regulating signaling pathways originated within these membrane regions.