Abstract
Sphingolipids constitute a class of lipids defined by their eighteen carbon amino-alcohol backbones which are synthesized in the ER from nonsphingolipid precursors. Modification of this basic structure is what gives rise to the vast family of sphingolipids that play significant roles in membrane biology and provide many bioactive metabolites that regulate cell function. Despite the diversity of structure and function of sphingolipids, their creation and destruction are governed by common synthetic and catabolic pathways. In this regard, sphingolipid metabolism can be imagined as an array of interconnected networks that diverge from a single common entry point and converge into a single common breakdown pathway.
In their simplest forms, sphingosine, phytosphingosine and dihydrosphingosine serve as the backbones upon which further complexity is achieved. For example, phosphorylation of the C1 hydroxyl group yields the final breakdown products and/or the important signaling molecules sphingosine-1-phosphate, phytosphingosine-1-phosphate and dihydrosphingosine-1-phosphate, respectively. On the other hand, acylation of sphingosine, phytosphingosine, or dihydrosphingosine with one of several possible acyl CoA molecules through the action of distinct ceramide synthases produces the molecules defined as ceramide, phytoceramide, or dihydroceramide. Ceramide, due to the differing acyl CoAs that can be used to produce it, is technically a class of molecules rather than a single molecule and therefore may have different biological functions depending on the acyl chain it is composed of.
At the apex of complexity is the group of lipids known as glycosphingolipids (GSL) which contain dozens of different sphingolipid species differing by both the order and type of sugar residues attached to their headgroups. Since these molecules are produced from ceramide precursors, they too may have differences in their acyl chain composition, revealing an additional layer of variation. The glycosphingolipids are divided broadly into two categories: glucosphingolipids and galactosphingolipids. The glucosphingolipids depend initially on the enzyme glucosylceramide synthase (GCS) which attaches glucose as the first residue to the C1 hydroxyl position. Galactosphingolipids, on the other hand, are generated from galactosylceramide synthase (GalCerS), an evolutionarily dissimilar enzyme from GCS. Glycosphingolipids are further divided based upon further modification by various glycosyltransferases which increases the potential variation in lipid species by several fold. Far more abundant are the sphingomyelin species which are produced in parallel with glycosphingolipids, however they are defined by a phosphocholine headgroup rather than the addition of sugar residues. Although sphingomyelin species all share a common headgroup, they too are produced from a variety of ceramide species and therefore can have differing acyl chains attached to their C-2 amino groups.
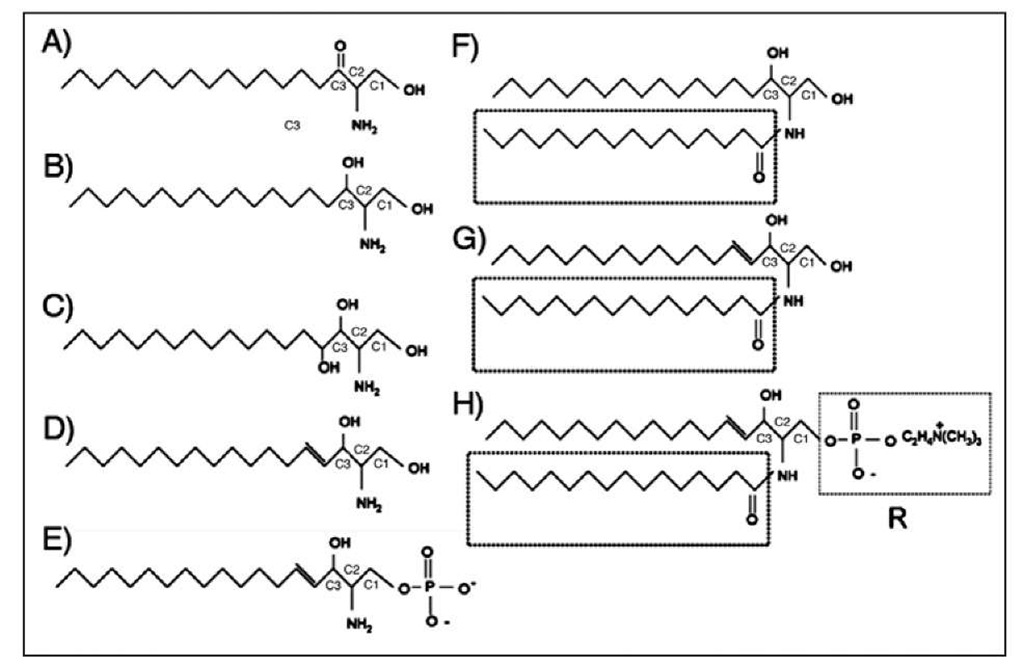
Figure 1. Simple and complex sphingolipid structures. Structures shown: (A) 3-Ketodihydrosphin-gosine, (B) Dihydrosphingosine, (C) Phytosphingosine, (D) Sphingosine, (E) Sphingosine-1-Phosphate, (F) Dihydroceramide: Boxed region shows variable acyl chain, (G) Ceramide, (H) Complex Sphingolipids: Sphingomyelin shown with phosphocholine R group. Substitute R for glucose=Glucosylceramide, Substitute R for galactose=Galactosylceramide.
Whether or not the differing acyl chain lengths in SMs dictate unique functions or important biophysical distinctions has not yet been established. Understanding the function of all the existing gly-cosphingolipids and sphingomyelin species will be a major undertaking in the future since the tools to study and measure these species are only beginning to be developed (see Fig 1 for an illustrated depiction of the various sphingolipid structures).
The simple sphingolipids serve both as the precursors and the breakdown products of the more complex ones. Importantly, in recent decades, these simple sphingolipids have gained attention for having significant signaling and regulatory roles within cells. In addition, many tools have emerged to measure the levels of simple sphingolipids and therefore have become the focus of even more intense study in recent years. With this thought in mind, this topic will pay tribute to the complex sphingolipids, but focus on the regulation of simple sphingolipid metabolism.
Sphingolipid Properties in Membranes
An important feature of lipid biology is that many of these molecules are restricted to biological membranes and therefore lipids are governed by a set of rules based on their biophysical properties. For example, compartmentalization of a lipid can mean access to both sides of a membrane, access to only a single leaflet of a bilayer and therefore only a single compartment, or a molecule could be sufficiently amphipathic that it could diffuse from a membrane and freely traverse the cytosol, the lumen of an organelle, or enter the extracellular space. This becomes important to keep in mind when understanding how compartmentalized enzymes only have effects on a specific pool of lipid metabolites. It is also important to understand the enzymes that regulate the lipids since cytosolic proteins are generally restricted to a single compartment and transmembrane domain containing enzymes generally have their catalytic sites facing only one of the leaflets in which a bilayer divides.
In the case of sphingolipids, sphingosine and dihydrosphingosine are sufficiently amphipathic to diffuse between membranes and to flip between membrane leaflets; however, they also are likely to accumulate in acidic pH organelles due to ionization of their free amino group. All of the known enzymes to act upon sphingosine have their catalytic sites facing the cytosolic compartment suggesting that only cytosolic sphingosine is in a modifiable form. Ceramide, on the other hand is restricted to membranes, but has a relatively rapid flip rate. Ceramide is therefore likely to be restricted to the organelle in which it was created, but may have access to enzymes or binding proteins on either side of the bilayer in which it was produced. This is important because these enzymes are distributed in discrete compartments and therefore ceramide will most likely be modified by whichever enzyme is within the same compartment in which the ceramide was generated or transported to. Sphingomyelins and glycosphingolipids are the most spatially restricted sphingolipids of all since their bulky head-groups make flipping between membrane leaflets extremely unlikely without the aid of specific flippases. One such flippase is thought to be present in the Golgi apparatus to aid glucosylceramide in gaining access to lumenal glycosyltransferases. In the absence ofa flippase, sphingomyelin and glycosphingolipids are restricted to whichever leaflet they are generated in and since they are generated in the Golgi lumen, they are mainly present in the lumenal Golgi leaflet or on the outer leaflet of the plasma membrane after vesicular transport to that location. Finally, the ultimate catabolic products of all sphingolipids are sphingosine-1-phosphate and dihydrosphingosine-1-phosphate which are soluble in a hydrophilic environment, but are unable to traverse membranes without the aid of lipid transporters. Therefore, S1P and DHS1P are also restricted to the hydrophilic compartments in which they are generated, but can be exported to the extracellular space with the aid of specific transporters or be dephosphorylated into a more hydrophobic compound.
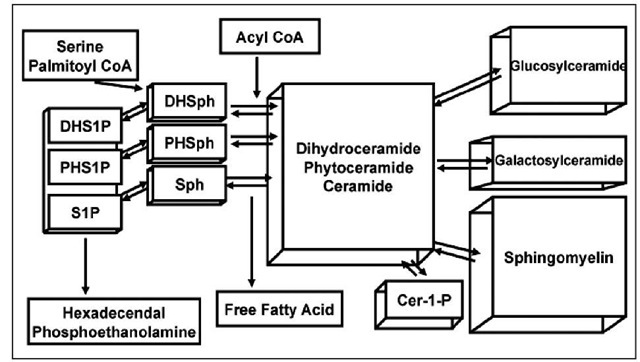
Sphingolipid structures differ significantly from kingdom to kingdom and sphingolipid diversity within the animal kingdom itself has been recognized. Despite this diversity being extremely fascinating for its implications in evolutionary biology, this topic will only discuss mammalian enzymes involved in sphingolipid metabolism. Although many of the critical enzymes in mammalian systems could not have been identified without the aid of yeast and other model organisms, for the sake of brevity, unless otherwise stated, the enzymes discussed in this topic are to be assumed to be the mammalian form. In addition, the reader may find it useful to utilize figure 2 as an illustrated reference of sphingolipid metabolism as they progress through this topic. By discussing the synthesis and the catabolism of mammalian sphingolipids we hope to bring to light an understanding of sphingolipids, how sphingolipids are created and destroyed and to define more clearly the process by which sphingolipids become distributed to their respective membranes within mammalian cells.
De Novo Synthesis in the ER
De novo sphingolipid synthesis begins at the cytosolic leaflet of the ER where a set of four enzyme groups coordinately generate ceramides of different acyl chain lengths from nonsphin-golipid precursors. Through the coordinated action of Serine palmitoyltransferase, 3-Ketodihy-drosphingosine Reductase and (dihydro)Ceramide Synthases, the ER is able to convert cytosolic serine and palmitoyl CoA molecules into a single membrane bound lipid, dihydroceramide. After its generation, dihydroceramide is acted on by a desaturase which introduces a double bond. This coordinated anabolic pathway generates the precursors to complex sphingolipids that can serve such diverse functions as providing electrical insulation to axons, act as an important hydrophobic barrier within the epidermis essential for decreasing water loss and regulate red blood cell surface charge to prevent agglutination, to name just a few important functions.
In this phase of synthesis, complex sphingolipids begin to be diversified through differential addition of fatty acyl chains at the C2-amino group ofthe dihydrosphingosine/sphingosine backbone through the action of ceramide synthases. Variations in ceramide acyl chain length as well as the use of alpha hydroxylated fatty acids could potentially alter membrane bilayer dynamics, or have differential signaling properties by recruiting different binding partners. The effects of different acyl chain lengths on ceramide or complex sphingolipid biology are not yet understood.
Serine Palmitoyltransferase and 3-Ketodihydrosphingosine Reductase
The initial reaction in sphingolipid synthesis requires the enzyme serine palmitoyltransferase (SPT). This reaction occurs through cytosolic serine and palmitoyl CoA condensation to produce 3-ketodihydrosphingosine.1 SPT is encoded by the genes SPTLC1, SPTLC2 and the recently identified SPTLC3.1 Each SPT subunit contains several putative transmembrane domains and displays Type I topology with its N-terminus directed into the ER lumen, C-terminus facing the cytosol and its catalytic site facing the cytosol.3 SPT1 and SPT2 form a heterodimer in the ER membrane which is likely the active form of the enzyme.4 Although SPT3 shares 40% homology to SPT2 and likely can substitute for the SPT2 subunit in the SPT complex, it has not yet been shown to dimerize with SPT1 and still needs further investigation.2 In yeast, a third subunit of SPT, TSC3 plays a major role in regulation of SPT activity by forming a heterotrimer with the SPT1 and SPT2 homologues, however, no mammalian homologue to TSC3 has yet been identified.5 SPT is a member of the a-oxoamine synthase family, a group of enzymes that catalyze the condensation of amino acids with carboxylic acid CoA thioesters.6 Like other members of this family, SPT requires the cofactor pyridoxal 5′-phosphate (PLP) for catalysis.7 SPT2 is the only subunit which binds PLP, however both subunits are required for catalytic activity.4 Hanada et al have proposed that SPT2 requires SPT1 for stabilization and that up regulation of SPT1 expression subsequently leads to an increase in SPT2 expression through protein stabilization. Therefore, a secondary function of SPT1 other than its contribution to catalytic activity may be simply to stabilize SPT2 in the ER.6 Whether or not regulation of SPT1 is the primary means by which SPT activity is regulated is yet to be determined.
Some insight into the functional significance of SPT comes from human patients with Hereditary Sensory and Autonomic Neuropathy Type I (HSNI), a disease which, in some families, has been mapped to a mutation in the SPTLC1 gene on chromosome 9.8 HSNI is an autosomal dominant disease that is characterized by progressive degeneration of motor neurons and dorsal ganglia with symptoms initiating after the first or second decade.9,10 Measurements oftotal SPT activity in patient lymphoblasts showed less than 50% SPT activity within these cells.11 It is significant that patients still have a significant amount of SPT activity since in mice, complete lack of either SPT1 or SPT2 was shown to be embryonic lethal.4,12 The mutations responsible for this disease have been identified as point mutations in Cys133 or Val144 in the SPT1 protein which act in a dominant negative fashion on SPT activity.8,11 These mutations have been predicted to be localized, based on tertiary modeling of other known a-oxoamine synthases, near the catalytic interface of SPT1 and SPT2. HSNI mutant forms of SPT1 are able to form heterodimers with SPT2, but lack catalytic activity.13 Interestingly, mutations in the SPTLC2 gene were not identified in any ofthe tested families affected by HSNI.14 The recently identified SPTLC3 gene product has homology to SPTLC2 suggesting that SPT3 may be able to functionally substitute for SPT2 if expressed.2 It is possible that a functional SPT3 subunit could mask a partial defect in the SPT2 subunit if they are functionally redundant. Although speculative, this could be one explanation for why SPTLC2 mutants are not associated with HSNI. Future studies into the interplay between the three SPT subunits will provide further insights into how SPT function is determined by its components.
The second step in the synthesis of all sphingolipids is performed by the enzyme 3-Ketodihydrosphingosine Reductase (KDHR). 3-Ketodihydrosphingosine (KDHSph), the direct product of SPT is reduced at its ketone group to a hydroxyl group by KDHR in a NADPH dependent manner. Only recently were the human and murine genes cloned for this enzyme based on a homology screen for the yeast gene TSC10. TSC10 deficient yeast were identified based on their build up of KDHSph and their inability to grow on media deficient in KDHSph.15 In humans the KDHR gene was identified through a homology screen as FVT-1, a gene which was originally identified and named for its juxtaposition to the Ig-K gene in a human follicular lymphoma. 16 Whether or not the FVT-1 translocation was merely coincidental or that it conferred any advantage to the follicular lymphoma is unclear. KDHR is predicted to have three transmembrane domains and display Type I topology. Like SPT, KDHR has its catalytic site on the cytosolic leaflet of the ER where it is likely to encounter newly generated KDHSpH.16 KDHSph is a minor lipid within cells due to the rapid conversion of KDHSph into dihydrosphingosine by the action of KDHR. Although poorly studied, KDHR is a critical step in the synthesis of sphingolipids. Its importance to mammalian physiology has been highlighted by a breed of cattle, recently identified with a missense mutation in FVT-1, that become afflicted with bovine spinal muscular atrophy and die shortly after birth.17
Dihydroceramide Synthases/Ceramide Synthases and Dihydroceramide Desaturase
Dihydrosphingosine (DHSph) is further acylated by the action of six distinct (Dihydro) ceramide synthases. In mammals, six distinct (dihydro)ceramide synthases abbreviated as CerS1-6 have been identified and are encoded by six distinct genes.18,19 No other step in sphingolipid metabolism has as many genes devoted to it as dihydroceramide synthesis, suggesting that the different CerS have distinct functions. There is a significant amount of evidence that each CerS has a distinct, but overlapping acyl CoA preference and that each CerS can produce different dihydroceramide/ceramide species profiles. For example, CerS1 has been shown to prefer stearoyl CoA as a substrate and mainly produces C18-ceramide species.20 On the other hand, CerS2 utilizes C20-C26 acyl CoA species and is one of the major CerS responsible for very long chain ceramide species.21 Cers5 and CerS6 both prefer palmitoyl CoA as substrates and generate predominantly C16-ceramide species.22,23 Finally, CerS3, which is predominantly expressed in the testis and weakly in the epidermis, prefers middle and long chain acyl CoAs and is thought to be a contributor to large structural sphingolipid molecules that maintain the water barrier in the epidermis.24,25 It is still an outstanding question whether or not the bioactive properties associated with ceramide are sensitive to differences in acyl chain length and hence different CerS can influence the cellular fate of cells by modulating bioactive molecules. Another possibility is that the six different CerS exist because differences in the biophysical properties of various ceramide species provide advantages for specific tissue functions (e.g., epidermal barrier maintenance or myelination). It has already been established that the CerS have a significant variation in their tissue expression and that this correlates with differences in their sphingolipid acyl chain compositions.21
All CerS studied to date have been localized to the ER with their catalytic sites facing the cytosol. In this manner, CerS are in a position to acylate newly generated DHSph molecules at their C2-amino groups in the presence of available fatty acyl CoAs.
Very little is known about the regulation of CerS activity in cells although there are clear differences in CerS expression between different tissues. It is unclear if CerS are predominantly regulated at the transcriptional level or if significant posttranslational regulation also occurs. Recently, a S1P binding site was identified on CerS2, which in vitro inhibited CerS2 activity. This suggests that sphingolipid breakdown could negatively regulate a specific subset of CerS activity within cells, in this case very long chain ceramide synthesis. Although de novo synthesis of dihydroceramide has repeatedly been shown to occur in response to various stress stimuli, the mechanisms by which this occurs remain opaque. Those that have studied it have suggested that the regulation is post-translational (e.g., not inhibited by cycloheximide).26
Although the family name of sphingolipids was named after the molecule sphingosine, this molecule is not actually generated during de novo synthesis. Only through the desaturation of dihydroceramide is the molecule sphingosine eventually generated.
Dihydroceramide A4-desaturase (DES) is the member of the desaturase family responsible for converting the dihydrosphingosine backbone within ceramide into a sphingosine backbone.27 DES utilizes molecular oxygen to first introduce a hydroxyl group into the C4 position of the dihydrosphingosine backbone and following a dehydration reaction, with the aid of NADPH, produces a double bond in the C4-C5 position of dihydroceramide.28-30 Dihydroceramide with a double bond introduced at this position is referred to as ceramide. Dihydroceramide A4-desaturase (DES1), encoded by the DES1 gene contains multiple transmembrane domains and was recently shown to require myristoylation on its N-terminus for full activity.
Figure 2. The sphingolipid metabolic network.
31,32 Like the previous three enzymes in the sphingolipid biosynthetic pathway, DES1 is embedded in the ER membrane where it has access to newly synthesized dihydroceramide species.27 It is interesting to note that an intermediate reaction product in the conversion of dihydroceramide to ceramide is 4-hydroxyceramide which is also known as phytoceramide. Phytoceramide is the predominant ceramide species in plants and yeast. Although the enzyme DES1 only converts dihydroceramide species into fully desaturated ceramide, a second family member, dihydroceramide C4 hydroxylase/A4-desaturase (DES2), is capable of creating either phytoceramide or ceramide from dihydroceramide precursors.33 Therefore, it is not surprising that DES2 is highly expressed in the intestines, kidneys and skin where phytoceramides are present in high abundance.34,33 The differences in biophysical properties between dihydroceramide, ceramide and phytoceramide are not entirely clear, however, one may speculate that the addition of a hydroxyl group into the sphingosine backbone may increase lipid packing in the membrane by increasing the amount of hydrogen bonding at the interfacial region of the membrane. It has been shown repeatedly that ceramide has distinct signaling properties from dihydroceramide and phytoceramide, suggesting that, if nothing else, cells have evolved to recognize ceramide as a more significant determinant to initiate a cellular response to in most cells.35 The recent report of the phenotype of the Des1-/- mice suggests that the inability to form ceramide leads to serious consequences for mammalian physiology. Des1-/- mice have highly elevated dihydroceramide, low levels of ceramide, multi-organ dysfunction and failure to thrive.36
Ceramide Transport from the ER to the Golgi
Ceramide is a membrane bound molecule that has very low solubility in an aqueous environment and therefore, a cell must find a way to transport it from one membrane to another. The cell employs two major mechanisms to mobilize ceramide; either through vesicular transport or through the protein ceramide transfer protein (CERT). CERT is a cytosolic protein that transfers ceramide from the ER, where it is generated, to the Golgi apparatus where it can be modified into sphingomyelins and possibly glycosphingolipids. The CERT protein is composed of at least four functional domains that determine its function. The N-terminus of CERT contains a PH domain which is able to recognize PI4P on acceptor Golgi membranes and therefore allows for directed transport to the Golgi. A FFAT domain in the middle of the protein serves an analogous function to the PH domain but for donor membrane recognition. The FFAT domain is thought to allow its binding to ER resident VAP proteins and therefore CERT can only accept ceramides from the ER, something that may have implications for cellular signaling.37 The C-terminus of CERT contains a START domain which provides a hydrophobic pocket responsible for the direct binding of ceramide and allows for its delivery to the Golgi through an aqueous environment. In vitro studies with CERT have shown that phosphorylation of CERT at multiple serine residues, by an unidentified kinase, result in an autoinhibitory binding event that occurs between both the START domain and the PH domain.38 The in vivo significance of CERT phosphorylation is unclear and remains to be seen. On the other hand, a globular domain between the PH and START domain has been shown to be responsible for homotrimer formation during UV stress in keratinocytes, however, this was shown to be phosphorylation independent. It is unclear if oligomerization, or potentially phosphorylation, is a general mechanism by which cellular stresses can inactivate CERT.
CERT was originally identified as the responsible mutant in a CHO cell line, LY-A, that was resistant to hemolysis by the sphingomyelin-dependent celomate toxin lysenin .40,41 CERT displays a preference for ceramide species with acyl chains less than C22. Although CERT still transfers C22 and C24:1 ceramide, it does so with 40% the efficiency of shorter chain species.38,42 In addition, CERT showed minimal to no transfer of C24 ceramide. CERT is also able to recognize dihydroceramide and phytoceramide although less effectively than ceramide.38 Ceramide which is transported to the Golgi by CERT is preferentially incorporated into SM over glycosphingolipids.41 Since CERT has preference for specific chain lengths, this may have implications for which forms of ceramide are preferentially utilized for SM synthesis and which ceramide species are preferred for glycosphingolipid utilization. If this is true, then one could speculate that relative SM and glycosphingolipid synthesis could be regulated by shifting CerS expression from predominantly long chain specific CerS to very long chain specific CerS and vice versa.
An alternative pathway exists for the transport of ceramide species to the Golgi which is coatomer protein dependent and is based on vesicular transport.43 Less is known about how this pathway is regulated, however, this is thought to be the major pathway responsible for delivering ceramide to the cis-Golgi for glycosphingolipid synthesis. Clearly, our knowledge ofhow ceramide species can be transported from the ER to the Golgi for regulated glycosphingolipid synthesis is incomplete.
Synthesis of Complex Sphingolipids
Complex sphingolipids are divided into three major groups based on the primary residue attached to their C1-hydroxy headgroup. This classification also captures the three biosynthetic pathways, spatially separated within the ER and the Golgi complex, that generate an immense diversity of glycosphingolipids and sphingomyelins. The three major enzymes that regulate complex sphingolipid biosynthesis are ceramide galactosyltransferase, glucosylceramide synthase and sphingomyelin synthase.
Ceramide Galactosyltransferase and Galactosphingolipids
Ceramide galactosyltransferase (CGT) utilizes UDP-galactose and ceramide to create galactosylceramide. CGT is an ER transmembrane protein that has its catalytic site facing the lumen of the ER. It is structurally related to UDP-glucuronosyltransferases, an enzyme critical to Type II biotransformation of xenobiotics and porphyrin metabolism.44 CGT has a limited tissue distribution with expression being detected primarily in schwann cells, oligodendrocytes, kidneys, testis and intestines. In the central nervous system, the product galactosylceramide (and its subsequent metabolite, sulfatide) is highly enriched in myelin. CGT knockout mice display a tremor phenotype, severe motor weakness due to loss of nerve conduction, male infertility and premature death.45,46 Interestingly, the neuronal phenotype in mice lacking CGT can be rescued by expression of an oligodendrocyte specific CGT gene suggesting that galactosylceramide is extremely important for oligodendrocyte function.47 Galactosylceramide is a precursor for sulfati-des and many of the myelination defects may be due to a lack of sulfatide production. Evidence for this comes from mice deficient in the enzyme galactosylceramide sulfotransferase, the enzyme responsible for sulfatide production from galactosylceramide, which have major defects in myeli-nation, although their pathology is less severe than an outright CGT knockout mouse.48
Glucosylceramide Synthase and Derivatives of Glucosylceramide
Glucosylceramide is synthesized in the cis-Golgi from ceramide and UDP-glucose by the enzyme glucosylceramide synthase (GCS).49 GCS is a transmembrane protein present on the cis-Golgi and it has its catalytic site facing the cytosol where newly produced glucosylceramide can be recognized by the lipid transport protein FAPP2.50,51 Some reports suggest that FAPP2 transports glucosylceramide back to the ER where it is translocated from the inner leaflet to the outer leaflet, however, this point remains to be resolved.
Unlike galactosylceramide, glucosylceramide (GC) is an absolutely essential sphingolipid for the development of mammals.52 Mice lacking GCS do not survive to term. The loss of GCS results in embryonic lethality at embryonic day 6.5-7.5 when gastrulation is occurring.52 This specific defect can be rescued by the addition of exogenous GC to the embryos. Glucosylceramide is the precursor for the majority of all glycosphingolipids that can be produced by a mammal and these glycosphingolipids are likely to play an essential role in cell-cell recognition during embryonic and postnatal development.52
Tissue specific knockouts of GCS within the nervous system and the skin have been created. Absence of GCS in the epidermis leads to defects in lamellar body formation which are major contributors to the hydrophobic barrier of the skin. These lamellar body defects lead to rapid water loss due to excessive evaporation and eventual lethality several days after birth.53 Absence of GCS specifically in neuronal tissue, on the other hand, leads to premature death 11-24 days after birth, suggesting that glycosphingolipids are necessary for neuronal function and proper brain maturation. Unusually, no histological defects could be identified in the brains of the neuron specific GCS knockout mice through light microscopy examination or electron microscopic evaluation of synapses despite their obvious phenotypic differences. However, in vitro studies of primary hip-pocampal neurons from GCS deficient mice showed defects in neurite outgrowth in culture.54,55
Sphingomyelin Synthesis
The most abundant complex sphingolipids in mammalian cells are the sphingomyelin species. Evidence for the essential role that sphingomyelin has in eukaryotic cell viability is displayed by the inability of mammalian or yeast cells to survive in culture when they are unable to produce sphingomyelin either through CERT mutation or defects in de novo sphingolipid synthesis. It is interesting to note that this absolute requirement for a sphingolipid is not true for glucosyl-ceramide or galactosylceramide which, although critical for mammalian development and tissue specific functions are not required for the viability of cells in culture. The precise single function that sphingomyelin fulfills which is absolutely necessary for cell survival is not clear due to sphingomyelin’s many known functions in membrane biology.
Sphingomyelin is produced by the action of sphingomyelin synthases. There are at least two members of the sphingomyelin synthase family in most mammalian species56 and possibly a third family member known as SMSr.57The sphingomyelin synthases (SMS) are evolutionarily similar to the lipid phosphate phosphatase family which have six transmembrane domains and have their catalytic domains facing the luminal or exoplasmic leaflet of the membrane.57 Like their cousins, the SMS family constituents also have six transmembrane domains and are oriented with their catalytic sites facing the Golgi lumen or extracellular space. Sphingomyelin synthases 1 and 2 are both present in the trans-Golgi, however, SMS2 is also localized to the plasma membrane. Therefore, SMS2 may also have a unique function in maintaining plasma membrane sphingomyelin content directly at the plasma membrane. Generation of sphingomyelin occurs through the transfer of a phosphocholine headgroup from phosphatidylcholine to ceramide yielding the products diacylglycerol (DAG) and sphingomyelin (SM). Since both ceramide and DAG have been identified as bioactive lipids with opposing effects on cellular proliferation and survival, SMS has also been proposed to play an essential role in regulating cellular fate. A recent study has implicated SMS in generating DAG in the Golgi with effects on PKC.58 Because SMS activity directly regulates the level of sphingomyelin, ceramide, DAG and PC simultaneously, direct effects of SMS products on biological processes have been difficult to elucidate to date.