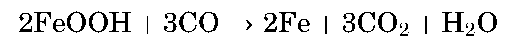Space resources consist of all of the useful materials, energy sources, and energy found in space. For practical purposes, this article focuses on those resources that may be accessible within the next 25 years, and for which large-scale demand is plausible. Therefore, the principal focus of this review is the bodies of nearby space: Earth’s Moon, the planets Mercury and Mars, the two small Martian moons, Phobos and Deimos, and the near-Earth population of asteroids and comets.
This article briefly surveys the significance and promise of space resources, including scientific and technical issues and the legal and regulatory regime surrounding their economic use. Current treaty obligations are reviewed, and some suggestions made regarding legislation and treaty language that would encourage the growth of this enormous new arena of economic activity, making vast new resources available to all.
Why Use Space Resources?
The cost of access to space is presently very high. To lift one kilogram from Earth’s surface into low Earth orbit costs about $10,000: a tonne of water aboard a space station represents an investment of nearly $10,000,000 in launch costs. Soft-landing one tonne of payload on the Moon costs about $100,000,000. A gallon of gasoline on the Moon would cost $400,001—$1 to purchase the gas on Earth and $400,000 to deliver it to the Moon.
Such a pricing system seems intuitively absurd: is there no better way to make intrinsically cheap, common, and highly desired materials available in space at affordable prices? How could we do better than launching them from Earth? Certainly any source of water, life-support materials, or propellants already present on the Moon or Mars would be enormously attractive. Even if it cost us $100 to extract or manufacture a liter of propellants on the surface of another planet, that amount would represent a 99.9% cost reduction, compared to transporting those propellants from Earth. We could then carry out the same mission for 0.1% of the propellant cost of launching the propellants from Earth— or move 1000 times as much payload for the same cost. To exercise this option, we require a scientifically, technically, and economically sound extraterrestrial source of propellants. This principle also applies to other commodities that are intrinsically cheap and in great demand in space, such as metal construction materials for building large space structures.
In recent years a number of suggestions have been offered regarding economic exploitation of the energy and material resources in nearby space. Among the resources proposed for exploitation are
1. solar energy, from space-based photovoltaic cells (Solar-Power Satellites or lunar power stations), via microwave transmission of power to antennae on Earth, to provide clean, cheap energy in vast amounts;
2. oxygen extracted from lunar or asteroidal rocks and minerals for use in life-support systems and as the oxidizer for rocket engines departing from the Moon;
3. the isotope helium-3, a constituent of the atmospheres of Uranus and the other giant planets and a very minor constituent of lunar regolith, for use as clean fusion fuel on Earth;
4. water from near-Earth asteroids, Martian permafrost, or lunar polar ice deposits for use in life support and rocket propellants;
5. metals, especially ferrous-metal alloys, for use in space construction; and
6. precious and strategic metals for importation to Earth.
As these examples suggest, the most important single motivation for space resource use is the recognition that large-scale future activities in near-Earth space may be made far less expensive by using materials that are already in space and that therefore need not be launched at great expense out of Earth’s gravity well. But this is not the only motivation. A second important benefit of using space-derived energy and resources is the enormous positive environmental impact of off-loading energy-related mining, drilling, and shipping and large-scale energy production from Earth’s surface. Return of valuable commodities to Earth merits third priority.
Many material resources found in space, especially on the Moon and Mars, would be used locally (i.e., within the gravity well of the body from which the resource was mined) to support unmanned and manned exploration or to fuel vehicles returning to Earth. Because of the substantial gravity fields of Mars and the Moon and the consequent high energy cost of landing and takeoff, as well as the low quality of their proven mineral resources, profitable export of materials from such massive bodies is far less feasible than local use: neither of these two bodies is well suited to export materials for use in other locations. For these logistical reasons and because of their great resource richness and diversity, near-Earth asteroids have emerged as the most attractive nearby sources of space-derived materials for export (especially, for return to near-Earth orbits).
The realization that these space resources may have enormous economic impact has stimulated considerable technical and economic interest. Two technical volumes on space resources have appeared in recent years, Space Resources (1) and Resources of Near-Earth Space (2). A popular overview of space resources based on these technical studies appears in Mining the Sky (3). A series of conference volumes containing papers on space resources, based on the proceedings of the biennial Princeton High Frontier Conferences and entitled Space Manufacturing (volume numbers I to XIV), has been published by the American Institute of Aeronautics and Astronautics. The technical literature on space resources is otherwise very widespread and difficult to research; it often appears in contractor reports, limited-circulation conference proceedings, and government publications. For this reason, using the volumes mentioned as introductions to the literature and as a source of citations to original publications is highly desirable. The Resources of the Moon. The best single source for information on the physical and chemical properties of the Moon is the Lunar.A detailed treatment can be found in the article, The Moon in this volume. General reviews of and references to processing of lunar materials are found in the basic references cited earlier.
The Moon is in orbit around Earth at a mean distance of 384,400 km from Earth’s center. Its orbit has an eccentricity of 0.055 and an inclination of 5.142° relative to the ecliptic that allows excursions of the Moon to as far as 28.7° north or south of the terrestrial equator. The Moon is airless and utterly devoid of liquid water. Its radius is 1738 km and its mass is 1/81.3 times the mass of Earth. Its surface gravity is 0.165 Earth gravities, and its escape velocity is 2380 m s ~1. The Lunar Rocks. The Moon is largely covered by heavily cratered highlands that are rich in the calcium aluminosilicate mineral, anorthite (CaAl2Si2O8), one of the two defining constituents of terrestrial plagioclase feldspar. The Moon is so severely depleted in alkali metals that the complement of sodium plagioclase (albite; NaAlSi3O8) normally found on Earth is reduced to a minor component on the Moon. The most common highland rock type is anorthosite, a rock dominated by anorthite. The second most abundant mineral in these rocks is generally a low-magnesium pyroxene, and olivine is also present. These rocks, the ferroan anorthosites, are among the most ancient rocks on the Moon and date back at least 4.4 Ga (billion years before the present).
The second most abundant highland rocks are magnesium-rich and compositionally diverse. They have plagioclase contents that range to nearly zero in dunite, which is nearly pure olivine rock. Olivine-plagioclase rocks are called norites, and pyroxene and Ca-poor plagioclase rocks are classified as gabbros or norites. Many of the Mg-suite highland rocks also date to before 4.4 Ga.
An unusual lunar highland rock type that contains enhanced abundances of potassium (K), the rare-earth elements (REE), and phosphorus (P), found only as small chips in the regolith, has been named KREEP. In major-element chemistry, KREEP is a basalt. As on Earth, the rare earths concentrate strongly in phosphate minerals. Gamma-ray spectroscopy conducted from lunar orbit during the Apollo program tentatively identified the KREEP source region on the edge of the
Mare Imbrium basin, based on the enhanced gamma emission from high concentrations of K, U, and Th. These data have recently been confirmed and refined by a gamma-ray spectrometer on the Lunar Prospector mission.
Chemical analyses of representative lunar igneous rocks show that most have silica abundances that are near the low end of the terrestrial range; they usually have less than 50% by weight of SiO2, within the range of terrestrial basalts. Materials from the lunar lowlands, which cover about 25% of the near side of the Moon, are typically basaltic in composition.
However, lunar basalts differ from terrestrial basalts in that the lunar rocks have alkali metal abundances that are several times lower and titanium abundances that are several times higher than the usual terrestrial examples. Because the very high titanium abundance is accounted for by large amounts of ilmenite (FeTiO3), the iron abundance is also abnormally elevated in lunar basalts relative to terrestrial basalts.
We find a range a range of textures, from the most fine-grained lunar basalts, which cooled rapidly upon extrusion as lava flows onto the surface of the Moon, to coarser grained basalts that crystallized more slowly and completely in a more protected (often intrusive) environment beneath the surface, forming volcanic sills or dikes or the bottoms of thick lava flows. Extruded basalts often show abundant vesicles, essentially bubbles inflated by magmatic gases such as hydrogen or CO, which unfortunately have long since diffused away.
Liquids of lunar basalt composition have very low viscosities and therefore are poorly suited for building tall volcanic structures about their magma vents. They are, however, ideal for making lava tubes, which may later drain and collapse to make features that look like the lunar rilles. These basaltic liquids have densities of only about 3.0, compared to about 3.3 for the solidified basalt. The observed bulk density of the Moon is only 3.34.
Lunar Minerals of Interest as Resources. Only seven minerals are ever found in abundances greater than about 1% in lunar rocks (Table 1). These include pyroxene (Ca,Fe,Mg)SiO3; calcic plagioclase (Ca,Na)(Al,Si)4O8, close to anorthite composition; ilmenite (FeTiO3); olivine (Mg,Fe)2SiO4; pyroxferroite CaFe6(SiO3)7; and two polymorphs of silica (SiO2), cristobalite and tridymite.
Pyroxene compositions in lunar rocks trespass into regions of the pyroxene quadrilateral not previously populated by other solar system materials. Some lunar pyroxenes are fairly standard augites, but others (often the outer layers of normal augite crystals) range far into the ferrosilite corner of the quadrilateral, perilously close to pure FeSiO3, which is thermodynamically unstable with respect to its component oxides. This new material crystallizes in the triclinic system, unlike monoclinic augite, and hence is given a new mineral name, pyroxferroite. Pigeonite, which is low in calcium, is also present in smaller amounts.
Feldspars are present as an unusually calcium-rich plagioclase (from about 60% up to 99% anorthite) and a potassium feldspar. The K-spar, which has a sanidine structure, is crystallized in very small amounts out of the residual melt in rapidly crystallized basalts.
Given the low silica content of most lunar rocks, the presence of small amounts of olivine is hardly surprising. Olivine is well approximated by a solid solution of Mg2SiO4 (forsterite; fo) and Fe2SiO4 (fayalite; fa). Most lunar olivine is fairly iron-rich (fa20-fa50), but virtually pure fayalite is sometimes observed.
Table 1. Selected Native Lunar Minerals
| Metals | ||
| Kamacite Taenite | Fe,Ni (<6% Ni) Fe,Ni (> 6% Ni)
Sulfides |
aa a |
| Troilite | FeS
Oxides |
a |
| Armalcolite Perovskite
Spinel S.S.b Spinel Hercynite Chromite Magnesiochromite Ulvospinel Cristobalite Tridymite Rutile Baddeleyite Ilmenite |
FeMgT12O5 CaTiO3
MgAl2O4 (Fe,Mg)Al2O4 FeCr2O4 MgCr2O4 Fe2SiO4 SiO2 SiO2 TiO2 ZrO2 FeTiO3 Oxysalts |
a a a
ma m a a M |
| Fluorapatite Chlorapatite
Whitlockite |
Ca5(PO4)3Fa Ca5(PO4)3Cl a Ca3(PO4)2 a | |
| Silicates | ||
| Olivine S.S.b
Fayalite Forsterite Pyroxene S.S.b Orthopyroxene Enstatite Ferrosilite Clinopyroxene Wollastonite Feldspar S.S.b Plagioclasex Anorthite Albite K-spar Orthoclase Sanidine Pyroxferroite Zircon |
(Mg,Fe)2SiO4
Fe2SiO4 Mg2SiO4 (Mg,Fe)SiO3 MgSiO3 FeSiO3 (Ca,Mg,Fe)SiO3 CaSiO3 CaAl2Si2O8 NaAlSi3O8 KAlSi3O8 KAlSi3O8 CaFe6(SiO3)7 ZrSiO4 |
m
Ma M a m a |
Fayalite, unlike pure ferrosilite, is thermodynamically stable. Many lunar basalts are close to or above silica saturation and often contain a few percent of cristobalite or tridymite or even a trace of quartz.
Ilmenite occurs in basalts at abundances from a few percent to more than 20%. There is an important difference in oxidation state between terrestrial ilmenite, which is a solid solution of FeTiO3 and Fe2O3, rich in ferric iron, and lunar ilmenite. Ferric iron is absent from lunar igneous rocks, and in fact small amounts of metallic iron are often found in lunar basalts. The ferric mineral FeOOH, ubiquitous in trace amounts in lunar samples, is an alteration product produced by the attack of terrestrial atmospheric water vapor on lunar lawren-cite (FeCy.
Metallic iron in lunar rocks, generally found in association with troilite, is nearly pure iron and contains less than 1% nickel. The molar ratio of metal to troilite always lies close to the eutectic composition for an Fe-FeS melt. On rare occasions, tiny traces of metallic copper are also found in association with the metal and troilite in basalts.
By contrast, metallic iron found in the regolith and in the shock-lithified microbreccia contains up to 30% Ni and 1% Co. The metal in the basalt seems to have been made by reduction of FeO during melting, whereas the regolith metal is clearly dominated by asteroidal debris. This view is reinforced by the common occurrences of traces of cohenite (Fe3C) and schreibersite [(Fe,Ni)3P], both common accessories of meteoritic metals, in association with Ni-bearing metal in the regolith and breccias.
Several interesting oxide minerals besides ilmenite are also found on the Moon (Table 1). Rare spinel, nearly stoichiometric MgAl2O4, has been found in breccias, chromite FeCr2O4 has been found within regolith nickel-iron particles of apparent asteroidal origin, and ulvospinel, nearly stoichiometric Fe2TiO4, has been found in trace amounts exsolved from or replacing ilmenite. Other titanates include armalcolite [(Fe,Mg)Ti2O5], usually found within ilmenite grains, and perovskite CaTiO3 that contains high concentrations of rare-earth elements, in the late-crystallizing component of the coarser basalts. Rutile TiO2 and both baddeleyite (ZrO2) and zircon (ZrSiO4) are also found in tiny quantities.
Few elements serve as important markers of the oxidation state of lunar material. We have seen lunar basalts lie close to the Fe-FeO buffer. Sulfur is fully reduced to sulfide (indeed, almost exclusively as troilite), and carbon is found as meteoritic carbide. Phosphorus, which is found as coexisting phosphide and phosphate in some meteorites, is found in accessory amounts in lunar basalts as apatite [Ca5(PO4)3X]. Fluorapatite (X = F) seems to be more common, but chlor-apatite (X = Cl) has also been reported. Hyroxylapatite (X = OH) has not been found. Small amounts of an amphibole that contains fluoride instead of hydroxyl have also been reported. Whitlockite [Mg3(PO4)2] that has very high concentrations of rare earths (to about 10%) has also been found as a component of KREEP. The Lunar Regolith. The lunar regolith, the crushed debris layer that covers the surface, is extremely complex. It is dominated by the products of violent cometary and asteroidal impacts on lunar igneous rocks and on the regolith itself. Rocks of all sizes, from house-sized boulders down to tiny rock chips and dust that contains only one or a few mineral grains, are mixed together with tiny glass droplets, similar to small chondrules, and with dust composed of very finely crushed grains. Impacts in the regolith have produced rocks, called microbrec-cias, that are essentially shock-lithified samples of the lunar regolith. Eclectic mixtures of smaller particles are often also found welded together by melt glass from later impacts. These welded, highly heterogeneous lumps are called agglutinates. In some places, the lunar regolith has been reprocessed so thoroughly that it has been mostly converted into agglutinates. Agglutinate-rich regolith samples are said to be mature.
Polar Volatiles on the Moon and Mercury. Lunar polar ice was apparently first envisioned as a resource by American rocket pioneer Robert H. Goddard in his student notebooks that date from 1908-1910. Harrison Brown and co-workers at Cal Tech in the early 1950s presented a simple quantitative argument for the preservation of ice from water-bearing impactors by recondensation in the lunar polar regions. These calculations were extended in the 1960s by James A. Arnold at UCSD, whose calculations stimulated considerable interest among both theorists and builders of spacecraft instrumentation.
The physical search for polar volatiles on the Moon and Mercury sounds at first like an exercise in futility. The problem is not just the expected very low abundance of volatiles in both bodies, but the logic of detection: the only places on the Moon and Mercury that are cold enough to permit trapping and long-term retention of volatiles (roughly 100 K for water ice) are permanently shadowed regions, such as crater bottoms, very close to the poles. Because the ice must be permanently shadowed to survive, it is always in the dark and cannot be photographed. Further, small, very cold regions on a generally very hot planet are difficult to detect in the infrared, where the long wavelengths of thermal infrared radiation degrade our spatial resolution and high fluxes from hot spots completely swamp the tiny fluxes from cold regions. The intensity of emitted thermal radiation from 600 K areas is greater than that from 100 K areas by a factor of (Thot/Tcold)4, or about 1300:1. Temperatures below 110 K are necessary to keep the evaporation rate low enough to preserve water ice for billions of years.
The first probe of the polar regions of Mercury was an ingenious experiment carried out in 1991 by Martin Slade of the Jet Propulsion Laboratory and Bryan Butler and Duane Muhleman of Cal Tech. They used the 70-meter radar transmitter at Goldstone in the Mojave Desert to illuminate Mercury with monochromatic (single-frequency) radar pulses at a wavelength of 3.5 cm. Slade and his coworkers transmitted a right circularly polarized (RCP) radar signal that would reflect from a flat mirror-like surface with left circular polarization (LCP). The center of the disk of Mercury is essentially a flat, though rough, mirror, so there is a large intensity spike in the returned signal that has minimum time delay, zero Doppler shift (after allowing for the relative motion of the transmitter and the center of Mercury), and strong left-hand polarization. Extremely complex scattering from a very rough surface tends to depolarize the returned signal, which means a relative increase in the RCP component. When the Goldstone/VLA data were analyzed, a bright RCP component was found at zero Doppler shift and maximum range, which requires that it originate at a pole. Fortunately, Mercury’s orbit is significantly tilted with respect to the plane of the ecliptic and affords observers on Earth frequent opportunities to see either pole. At the time of observation, the North Pole of Mercury was slightly tipped toward the observers and the South Pole was invisible, so the feature clearly must be associated with the north polar regions. Because the radar scattering properties observed for this feature are, so far as is known, unique to ice-covered surfaces, the strong implication is that Mercury has polar ice. Since these original observations, studies at different Earth-Mercury geometries have revealed that a similar feature is also present near the South geographic Pole. That area of anomalous reflection appears to be confined mostly to the floor of the large Chao Meng-Fu impact crater, which is centered at 87.5° S latitude.
Polar ice need not be exposed on the surface. Tens of centimeters of dry, porous dirt could overlie the ice without degrading the distinctive radar reflection signature of the ice. The thickness of the ice layer is poorly constrained by the observations. A few tens of centimeters or a few meters of ice would suffice to explain the observations, but a few kilometers of ice would be possible. Further, other materials besides water ice might possibly look the same.
Thermal modeling of the polar regions by David Paige and co-workers at UCLA shows that flat, unshadowed regions near the poles can be as cold as 167 K and that the observed cratering of the polar regions favors the existence of small, permanently shadowed enclaves inside craters that have large depth to diameter ratios. The craters that exhibit the highest depth to diameter ratios are simple bowl-shaped craters that have rim-crest diameters of 10 km or less. Unfortunately, because of the geometry of the Mariner 10 – Mercury encounters, our photographic (visible light) coverage of the polar regions has only an extremely narrow range of solar longitudes and leaves nearly half of the polar regions unimaged. The available coverage does, however, suggest areas that may be perpetually colder than 100 K, and even as low as 60 K. The coldest spot inside Saturn’s orbit may be on Mercury!
The knowledge that ices can be stable in the Moon’s and Mercury’s polar regions is fascinating but does not by itself tell us the source of the volatiles that are condensed there. Outgassing from their interiors seems an unlikely source if these planets are made of volatile-poor, high-temperature condensate; however, the mass of ice required to explain the observations may be as low as a few cubic kilometers, an amount so small as to make it impossible to rule out the presence of that much intrinsic water in the planet. Further, cometary impacts during billions of years provide vastly larger fluxes of water onto both bodies than the amount required by the radar data.
Long-period comet impacts can be enormously energetic events. At Mercury’s
long-period comet carries enough energy to eject its own mass, plus more than 740 times its mass of Mercury’s regolith, from the planet. This is not an efficient method of emplacing water on Mercury. Short-period comets, however, have average encounter speeds that are several times lower and have minimum encounter speeds of the order of 10 km s ~1. More important, extinct periodic comets (that have dust-insulated ice cores) and C-type planet-crossing asteroids may occasionally approach at velocities not much greater than escape velocity. Such impacts carry vastly less energy per gram and also have higher mean atomic weights in their fireballs, and hence expand more slowly. Thus water-bearing asteroids appear to be the most credible source of water and other volatiles on Mercury.
Unfortunately, our knowledge of the velocity distribution of Mercury-crossing asteroids is still very incomplete. Recent Space watch discoveries, mentioned later, show a surprising number of 10- to 500-m bodies in extremely Earth-like orbits of low eccentricity and modest inclination. The origin of this newly discovered class of bodies is not understood, and hence the likelihood of finding such a swarm of low-velocity bodies near Mercury cannot be assessed. Note also that, although there are many known Mercury-crossers among the near-Earth asteroid population, they represent an extremely biased set: all of them were discovered in the night sky from Earth and therefore must be in high-eccentricity orbits. Conversely, any low-eccentricity bodies that orbit near Mercury (or Venus) could not be discovered by present asteroid search techniques. Further, because all Mercury-crossing asteroids so far discovered must also cross Venus and Earth, they are strongly depleted in bodies whose inclinations are so low that they make frequent close approaches to these three planets.
One important implication of the discovery of polar ice on Mercury is that it suggested a similar phenomenon on the Moon. If there are massive quantities of polar volatiles on the Moon, both rocket propellants and life-support fluids could be manufactured readily on the lunar surface. Such a local source of propellants would greatly decrease the cost of launch operations from the Moon and possibly provide a source of propellant for export to other locations in the Earth-Moon system, most of which are far more accessible from the lunar surface than from the surface of Earth.
The first evidence of the presence of lunar polar ice deposits came from the Clementine mission in 1994. The Radio Science experiment on this spacecraft took advantage of the observed ”anomalous depolarization” of radio signals reflected from an ice surface, a phenomenon discovered in the course of radar studies of the icy Galilean satellites of Jupiter. The Clementine observations showed that when signals from the spacecraft transmitter are bounced at grazing incidence off the lunar poles, they were depolarized in the same way as those reflected from Europa, Ganymede, and Callisto—and the Mercurian poles. Although there is no convincing theoretical reason to link this phenomenon exclusively to water ice, it has not yet been shown that any other natural substance has the same effect. The data suggested that a small fraction of the surface area immediately adjacent to the lunar poles, possibly permanently shadowed crater bottoms, contains ice.
Clementine’s indirect detection of ice set the stage for the much more specific neutron spectroscopy carried out by the Lunar Prospector spacecraft. This experiment is diagnostic for the presence of abundant hydrogen. Identification of the hydrogen-bearing material as water ice is inferential, but ice is the most abundant hydrogen compound in the Universe and has a vapor pressure compatible with the deduced latitudinal distribution of ice on the Moon (and Mercury). To a chemist, the exact molecular speciation of hydrogen is of enormous interest, especially because these hydrogen compounds are almost certainly of cometary or asteroidal origin. But to a chemical engineer, it hardly matters which ices are present: reacting any of them with FeO at high temperatures releases water in abundance. Lunar Prospector found ice depositson crater floors near the poles with a total mass probably of the order of a billion tonnes (1015 grams). The ice is probably buried at a shallow depth in the regolith as interstitial ice, or permafrost, and partially fills the pore volume of the regolith.
Lunar Resource Exploitation. Many authors have suggested practical uses for lunar materials (Table 2). The simplest such use would be to employ regolith as radiation and micrometeoroid shielding for a lunar base. Using a sufficient power source, chemical processing of the regolith can also be attempted.
The most frequently discussed lunar product is oxygen, liberated by reduction of oxides of iron and possibly other metals (5). This process naturally makes metals, especially iron, available as an ancillary product. Ferrous oxide, an abundant and easily reduced component of many lunar minerals (especially pyroxene [(Mg,Fe,Ca)SiO3], olivine, [(Fe,Mg)2SiO4], and ilmenite (FeTiO3) can be reduced at elevated temperatures by gaseous reagents such as hydrogen, carbon monoxide, and methane. The oxygen-bearing product, water vapor and/or carbon dioxide, can then be split electrochemically to release oxygen and reconstitute the original reducing agent.
In principle, it would be desirable to heat and process only chemically pure mineral separates. However, beneficiation, the process of separating high-grade mineral fractions, is difficult on the Moon for a number of reasons. First, ”static cling” makes powders extremely sticky in the lunar vacuum and inhibits electrostatic or magnetic separation. Second, ilmenite-bearing rock chips have other minerals firmly bonded to the ilmenite, preventing clean separation. Third, mature regolith has been extensively processed into agglutinates, which weld together grains of disparate compositions. Fourth, the glassy component of the agglutinate is a mutual solution of many minerals that have been rendered inseparable by melting.
Table 2. Resource Targets on the Moon
| Product | Resource target | Process |
| Shielding | Regolith | Mechanical |
| Oxygen | Ilmenite | CO reduction H2 reduction Methane/HC reduction H2SO4 dissolution Li/Na reduction Plasma reduction |
| Mare regolith | H2 glass reduction Magma electrolysis Strong base solution Carbon reduction Vapor pyrolysis HF dissolution | |
| Highland rock | Magma electrolysis Strong base solution Carbon reduction | |
| Fe/Ni metals | Regolith | Magnetic separation Any reduction process Magma electrolysis |
| Ilmenite | Any reduction process | |
| Water | Regolith | Solar wind H release |
| Polar ice deposits | Melting, distillation | |
| Refractories | Regolith | Electrolytic residue |
| Ilmenite | Any reduction process | |
| Helium-3 | Ilmenite | Heating, gas fractionation |
If ferrous metals are sought, ilmenite reduction is a logical technique: the process (ignoring dross and unreacted ilmenite) produces oxygen, iron, and the refractory oxide, rutile (TiO2). Passing the iron through the gaseous carbonyl (Mond) process produces high-purity iron that is so free of defects that it resists corrosion as well as stainless steel.
Other processes for extracting oxygen, including high-temperature thermal decomposition of oxides, chlorination of ilmenite, whole-rock fluorination, and electrolysis of molten lunar material, also have attractive aspects but are not as well studied as hydrogen or carbon monoxide reduction. Of these, whole-rock schemes have the distinct advantage that they require only minimal physical processing and do not require beneficiation and sizing of selected minerals. They also share a disadvantage: any scheme that produces a variety of metals requires a much more complex chemical processing plant to turn the metals into useful products. Chlorination and fluorination processes also require very efficient recovery of the halogen reactants from a wide diversity of halide products.
Nonferrous metals are also abundant on the Moon. Titanium from the rutile by-product of ilmenite processing is an obvious possibility for extraction. Aluminum from anorthite and magnesium from pyroxenes and olivine should also be considered. Some authors have advocated using lunar calcium (also from anort-hite) for making electrical wires and cables for outside use in an environment free of water vapor and oxygen.
Perhaps the easiest source of ferrous metals is simple magnetic extraction of native metal grains from the regolith. These grains have two origins: nearly pure metallic iron, produced by reduction of FeO by implanted solar-wind hydrogen during impact shock-heating, and asteroidal iron-nickel-cobalt alloys left behind by the explosion of asteroidal impactors. Like polar ice and helium-3, these materials owe their presence on the Moon to external sources of hydrogen and metals: none are native to the Moon.
The use of lunar polar ice may be very difficult. It is stable only in permanently shadowed areas, so it is not easy to use sunlight to evaporate or melt the ice. If there are permanently illuminated mountain peaks adjacent to the ice deposits, one could envision a manned lunar polar base occupying that choice location, feeding solar power to a mining operation on the crater floor. However, even given such a fortuitous geographical arrangement, schemes for installing and operating a base and mine under such extreme conditions are very daunting. The installation of base modules requires landing in incredibly rugged terrain atop a lunar peak. Mining operations would have to be conducted at a temperature below about 100 K, a temperature so low that most metals would be brittle, and in the presence of pervasive fine, highly abrasive lunar dust. Ice at such low temperatures is as strong as rock.
Polar ice is not the only possible source of volatiles on the Moon. Solar-wind-implanted volatiles, especially hydrogen and helium, are widespread, especially in the equatorial region. These gases are implanted as ions in the surfaces of grains exposed atop the lunar regolith. Old, mature regolith has accumulated far more gases than young, immature regolith, from 10 to as much as 50 ppm (parts per million) by weight. The highest solar-wind gas concentrations are found in fine-grained ilmenite: it would be desirable to invent a beneficiation scheme that isolates ilmenite and screens out the largest grains. Unfortunately, mature regolith is very rich in agglutinates, which weld grains together irrespective of their composition, size, or gas content. Beneficiation of ilmenite or screening the ilmenite is impossible without thorough crushing to liberate individual grains. Further, the grains of greatest interest are so small that electrostatic “cling” makes it very difficult to separate them from grains of other sizes or compositions. Many simplified studies of ilmenite beneficiation have been reported; the most successful are those that least fit lunar conditions. The best recipe for success in liberating ilmenite is to separate magnetically, in air, a terrestrial ilmenite-bearing simulant free of agglutinates. But terrestrial ilmenite is a highly magnetic solid solution of iron oxides in ilmenite, not at all like lunar ilmenite. Success under such unrealistic conditions does not translate into success with unliberated, dust-like, slightly magnetic ilmenite in a vacuum.
The obvious method ofextracting implanted hydrogen and helium is to heat the lunar material. It is reasonable that a feedstock containing 50 ppm of hydrogen could be heated efficiently so that 40 ppm of hydrogen is recovered. Then 25,000 tonnes of regolith must be heated per tonne of hydrogen recovered.
G.L. Kulcinski and his collaborators at the University of Wisconsin have suggested the recovery of solar-wind-implanted helium-3 from the lunar regolith for return to Earth for use as a clean fusion fuel with terrestrial deuterium (6). Near-perfect recovery of helium-3 from mature regolith would require heating 100,000,000 tonnes of regolith per tonne of helium-3 extracted. Based on extremely generous assumptions regarding helium-3 content, efficiency of gas extraction and recovery, beneficiation and sizing of ilmenite particles, heat reclamation from the baked regolith, sealing the ovens gas-tight in the presence of ubiquitous dust, and the ability of great quantities of complex mechanical equipment to survive autonomous operation in a dusty hard vacuum with diurnal temperature extremes of — 200 to + 200°C, the scheme can be made to turn a handsome profit. Although it is far too early to identify this scheme as practical and economically attractive, it is not too soon to contemplate realistic tests of components of this proposed system in lunar simulators and even on the Moon itself.
Logistical Considerations. Outbound missions from low Earth orbit (LEO) to the lunar surface have total propulsive velocity requirements of 3.0 km s — 1 (from LEO nearly to escape velocity) plus 0.7 km s — 1 to match orbital speeds with the Moon and 2.4 km s — 1 to decelerate the spacecraft to a soft landing on the lunar surface, a total of 6.1 km s — 1.
Inbound missions from the lunar surface to a LEO Space Station require the same total velocity change; however, because the Space Station is so close to the top of Earth’s atmosphere, the 3.0 km s— 1 of braking required for the returning lunar vehicle to match speeds with the LEO Station can be provided by aerobraking, the controlled passage of the space vehicle, with an appropriate heat shield, through Earth’s upper atmosphere below the 100-km altitude level. The perigee of the resulting capture orbit, ranging from about 60-100 km, must then be lifted by an apogee burn to circularize the orbit at the same altitude as the LEO station. This maneuver typically requires 0.3 km s ~1 velocity change a total propulsive change of 3.3 km s ~1.
The Resources of Mars
In addition to the general topics on space resources suggested earlier, Martian resource exploitation is a major concern at the Case for Mars Conference series. The proceedings of these conferences are the basis of a series of topics starting with The Case for Mars (7-10). A popular account of planning for future manned Mars missions, including Mars resource exploitation, is found in the topic, Islands in the Sky (11).
Because of its substantial escape velocity, Mars is even less suited for export of products than the Moon. Nonetheless, production of propellants on the Martian surface is highly attractive. Manned missions would also benefit greatly from extracting life-support materials, such as water, oxygen, nitrogen, and nutrients from local resources. A demand for volatiles naturally motivates a search for methods of extracting these materials from the Martian atmosphere, rather than from crustal minerals.
Materials Available on Mars. Geochemically, Mars is intermediate in properties between Earth and the Moon. The presence of a water-bearing carbon dioxide atmosphere on Mars enables a wide range of weathering reactions and products never found on the anhydrous, anoxic Moon. Solar ultraviolet photolysis of both carbon dioxide and water vapor provides a continuous weak supply of oxygen, which accelerates the weathering process and makes a variety of highly oxidized surface minerals, notably ferric oxides and oxysalts such as carbonates and sulfates. Much of the Martian surface has experienced the direct action of liquid water in the distant past. Clay minerals and hydrated salts are certainly widespread, possibly ubiquitous, components of the surface. Nitrogen, a minor constituent of the atmosphere, may also contribute small quantities of nitrate minerals by photochemical production of nitrogen oxides.
Primary igneous rocks on Mars have been spectrally mapped from Earth and from orbit. The discovery that the rare shergottite, nakhlite, and chassignite (SNC) achondritic meteorites contain adsorbed gases that have the distinctive chemical and isotopic signature of the Martian atmosphere has led to the general acceptance that they are fragments of the Martian crust that have been hurled into independent orbits around the Sun by asteroid or comet impacts on Mars. Calculations by Ann Vickery and Jay Melosh of the University of Arizona have shown that rock fragments from shallow depths in the regolith may be accelerated to escape velocity by the blast wave from such impactors (12). The SNC meteorites, of which more than a dozen have now been identified, are igneous rocks that contain elevated concentrations of ferrous iron and traces of weathering products characteristic of the Martian environment.
Direct chemical analyses of the Martian surface dirt have been carried out by the Viking landers and the Mars Pathfinder mission. The dirt is characterized by high sulfur and iron and low potassium and sodium contents. This combination of factors is best understood as the result of oxidative weathering and hydration of a relatively low-temperature (FeO- and sulfur-rich) primitive material, followed by leaching and partial removal of water-soluble salts. In addition, traces of weathering products have been found in several of the SNC meteorites. Along with smectite- and illite-like clays, these products include sodium and potassium chlorides; hydrated ferrous oxides; and carbonates, phosphates, and sulfates of calcium and magnesium, some of which carry water of hydration.
The Martian poles are covered with deep, probably permanent caps of dust-laden water ice, often covered by frosts of solid carbon dioxide (”dry ice”). Because of the orientation of the line of apsides of the Martian orbit relative to the direction of the polar axis, the seasons are not symmetrical between the Southern and Northern Hemispheres: the colder (southern) polar cap presently retains solid carbon dioxide all summer. Seasonal precipitation of solid carbon dioxide (dry ice) snow occurs at high latitudes and altitudes. Ultraviolet spectra of the poles reveal the presence of a tiny trace of ozone in the ice deposit. The ozone is a minor product of atmospheric photochemistry.
Manufacture Of Propellants on Mars. The first use of Martian resources is likely to be to produce propellants to support unmanned or manned missions to the Martian surface, including use for both local mobility and for return to Earth (Table 3). In the longer term, manned missions may use native Martian materials to provide their requirements of air (oxygen and nitrogen) and water.
By far the most accessible and most easily ”mined” resource on Mars is atmospheric carbon dioxide. Ash et al., (13) first proposed that Martian carbon dioxide could be dissociated into carbon monoxide and oxygen by exposure to a hot (roughly 1000 K) zirconia membrane. By applying an electrical potential across the membrane, oxygen can be pumped selectively (as oxide ions), leaving a carbon dioxide/carbon monoxide mixture on one side and oxygen on the other
Table 3. Resource Targets on Mars
| Product | Resource target | Process |
| Shielding | Regolith | Mechanical |
| Oxygen | Atmosphere | CO2 cracking—ceramic membrane electrolysis |
| CO2 cracking—molten carbonate electrolysis | ||
| Fe oxides; FeOOH | CO reduction | |
| H2 reduction | ||
| Water | Electrolysis | |
| Fe metal | Regolith | Any reduction process, |
| Magma electrolysis | ||
| Water | Atmosphere | Condensation |
| Clays | Heating | |
| Permafrost/polar ice | Melting, distillation | |
| Refractories | Regolith | Electrolytic residue |
| Nitrogen/Ar | Atmosphere | CO2 removal |
Liquefaction of CO and oxygen provides a propellant combination that has a specific impulse of about 284 seconds, limited by both the modest enthalpy of combustion of CO and the high molecular weight of the exhaust (carbon dioxide; MW = 44).
At high pressures and moderate temperatures, carbon monoxide can disportionate via
to precipitate graphite, after which the carbon dioxide can be cycled through the zirconia cell to yield more oxygen.
A variety of additional propellants can be synthesized in the presence of other elements. If hydrogen is available, then hydrogen can be burned with oxygen (Isp = 460), or hydrogen can be used with carbon oxides to synthesize any of a variety of other fuels, including methane (CH4), methanol (CH3OH), ethanol (CH3CH2OH), and acetylene (C2H2). These fuels burn with oxygen to provide a lower specific impulse than hydrogen-oxygen but have the great advantage that they are noncryogenic. Some are liquid at the normal temperatures of the Martian surface. A discussion of schemes for integrating hydrogen with carbon-oxygen chemistry is found in Hepp et al. (14).
Hydrogen gas is not found on Mars, so taking advantage of these options requires finding a source of hydrogen. The two most obvious alternatives are to transport liquid hydrogen to Mars from Earth or to extract hydrogen from Martian water. The former requires carrying liquid hydrogen for several months, far beyond present experience for space missions. Martian sources of water include atmospheric water vapor, water of hydration in surface clays and salts, permafrost (at latitudes greater than about 45°), and polar ice (above roughly 80° latitude). The atmosphere of Mars, even when saturated with water, is so cold that the water content rarely exceeds a few parts per million. Although processing a clean gas stream appears attractively simple compared to mining, the energy cost of removing so small a trace of water appears prohibitive (15). The most concentrated source of water is polar ice, but accessing that ice requires operating during local summer when solar energy illuminates and warms the polar cap above the winter temperature of 140 K.
Permafrost is so widespread on Mars that it may prove almost as attractive as polar ice; however, permafrost may be buried under 0.1 to several meters of relatively dry regolith (16). Removal of the overburden may be easy, but mining permafrost requires extreme care because permafrost is effectively an abrasive-ice composite material. Finally, we consider extracting water from heated regolith that contains hydrated salts and hydrated and hydroxyl silicates, which is apparently possible anywhere on Mars. This approach may be the most direct and simplest to implement (17).
The second most attractive atmospheric component for propellant production is nitrogen. Nitrogen, combined with a chemical source for hydrogen and the ubiquitous carbon dioxide, allows synthesizing ammonia (NH3), hydrazine
(N2H4), hydrazine derivatives, and the storable oxidizers nitrogen tetroxide (N2O4) and nitric acid (HNO3).
Life-Support Materials on Mars. Water and oxygen have obvious direct application to life-support systems on Mars. Inert gases, especially nitrogen and argon, are also of considerable importance because of their use in admixture with oxygen to moderate the danger of fire. Together, nitrogen and argon make up about 5% of the Martian atmosphere.
Nutrients for plants and animals derive ultimately from inorganic materials. Beyond those materials already mentioned, chemically active nitrogen compounds such as ammonium and nitrate salts and phosphates deserve special mention.
Metals on Mars. Because of the high concentration of iron oxides in both Martian weathering products and primary igneous rocks, extraction of iron seems attractive (Table 3). The route to iron reduction could be either by carbon monoxide or solid carbon; the former is simpler and more practical. Carbon monoxide heated strongly with an iron oxide-hydroxide weathering product releases both carbon dioxide and water vapor and produces pure metallic iron:
Recovery of water and cycling of carbon dioxide through a cracking device allows oxygen recovery and CO regeneration. Reaction of carbon monoxide with iron or iron oxides at about 200°C and pressures of the order of 100 atmospheres produces a volatile iron carbonyl:
These moderate temperatures can be attained by passive solar heating without requiring electrical heating. The gaseous iron pentacarbonyl can then be passed over a heated surface at about 200°C and 1 atm pressure to decompose it quantitatively into high-purity metallic iron and gaseous carbon monoxide. This is a form of chemical vapor deposition. Iron deposited in this way is so pure that it has the corrosion resistance of stainless steel.
No other metal should be as readily extractable as iron. The very existence of aluminum ores appears improbable; indeed, if the SNC meteorites are representative, even plagioclase feldspar may be of low abundance locally. Titanium ores are also not established. The iron titanate mineral ilmenite, found in high concentrations in several lunar mare basins, is a minor or trace mineral in SNC meteorites. Chromite is also found in trace amounts. Other sulfides occur in minor or trace amounts, too low for reasonable economic exploitation, but suggesting the possible presence of undiscovered sulfide ore bodies on Mars (18).
It is important to note that existing data on Mars are of insufficient resolution and sensitivity to confirm or reject the presence of ore outcroppings of other economically useful materials. Hydrothermal deposits, either active or extinct, are almost certainly present. Therefore, ore mineralization must be considered a very real possibility. However, without knowledge of the nature and location of these ores, it is scarcely possible to suggest methods of use. It must suffice to point out that volatiles and iron, as discussed earlier, meet our most pressing local needs. Ancient sedimentary deposits might also provide calcium and magnesium carbonates, which suggest the possibility of manufacturing “Marscrete”, or Martian concrete, using carbonates that have been kilned to drive off carbon dioxide and make lime (CaO).
Logistical Considerations for Mars Missions. The total velocity requirement for outbound trips (from the LEO Space Station to the surface of Mars) is only 4.8 km s ~1, compared to 6.1 km s ~1 for missions from LEO to the lunar surface. The reason for the advantage enjoyed by Mars is that aerobraking for arrival at Mars is possible, and permits the dissipation of hyperbolic orbital energy without significant propellant consumption. The penalty paid for this liberation from heavy propellant use is that a heavy aerobrake heat shield must be carried.
The escape velocity of Mars, 5.4 km s ~1, is the mean of the escape velocities of Earth (11.2 km s ~1) and the Moon (2.4 km s ~1). Single-stage-to-orbit launch vehicles, which are marginally feasible on Earth, are highly appropriate for takeofffrom Mars. Because the most likely destination for a vehicle that departs from Mars is Earth and the entire impulse for injection into an Earth-intercept solar orbit can be delivered at launch while still deep in Mars’ gravity well, summing velocity requirements is incorrect: energy conservation gives a return velocity requirement of 7.8 km s ~1 for Earth intercept, compared to 3.0 km s ~1 for aerobraked return to Earth or LEO from the Moon. The returning Mars vehicle must then use aerobraking either to descend to Earth’s surface or to capture into Earth orbit.
Because of the relatively high escape velocity, it is probable that, aside from scientific samples and curios, export of Martian materials would be unprofitable.
The Martian Moons, Phobos and Deimos
The Martian moons are dark, irregularly shaped bodies whose characteristic dimensions are of the order of 10 km and masses of the order of 1018 grams (one trillion tonnes). Their orbits bracket Mars-synchronous orbit, so that Phobos passes from west to east across the Martian sky, whereas Deimos moves in the opposite direction. The masses and densities of these small satellites, measured by the Viking Orbiter spacecraft in the late 1970s, show that they are little more dense than ice and about half the density of even the least dense rocky meteoritic materials. Spectroscopic studies of the Martian moons have long suggested that they are very similar to the class of meteorites known as carbonaceous chondrites. Carbonaceous chondrites are black because of the pervasive presence of both tarry organic polymers and the iron oxide mineral, magnetite. They contain up to 6% of organic matter and up to 20% chemically bound water in the form of clays and hydrated salts. Spectroscopic studies in 1994, however, found that, unlike carbonaceous meteorites, Phobos and Deimos show no sign of the presence of water-bearing minerals.
One of the oddities of the Martian satellite system is its dynamic environment: the two satellites are so deep in the Martian gravitational field that most of the debris excavated from them by impacts remains in orbit about Mars. The debris fan from each impact quickly settles into a thin, dense dust disk in the equatorial plane, where it is eventually reaccreted by the two moons at low relative speeds. In the Asteroid Belt, for comparison, debris is injected into independent orbit around the Sun. It is dispersed over a vast volume of space and is subjected to severe disturbing forces from radiative pressure and gravitational perturbations, so that there is no reasonable probability that it will be reaccreted by the body from which it originated—or any other asteroid. Therefore, the surfaces of Phobos and Deimos, more than any asteroid, consist of materials ejected and strongly shock-heated in many generations of previous impacts. The surface material, not surprisingly, is very dry. But the surfaces of these bodies do not tell us whether their interiors are rich in water. The low observed density likewise is ambiguous evidence because it could be affected either by a large abundance of water ice or by pervasive fracturing of the interior: the insides of Phobos and Deimos may be full of both ice and vast systems of cracks and voids.
Utilization schemes for Phobos and Deimos clearly must await determination of their water content. If water is present and abundant, both satellites would become highly attractive as sources of propellants for use within the Mars system and for return to Earth.
Logistically, because of their low surface gravities, Phobos and Deimos are both more accessible for outbound soft-landing missions than the Moon. Return missions from Phobos and Deimos to Earth intercept require a very small velocity change to escape from the satellite, a larger DV to escape from Mars into heliocentric orbit, and another substantial DV to lower the perihelion distance of that orbit to 1AU. The total DV for return to Earth intercept (aerocapture) is 2.88 km s ~1 from Phobos and 2.55 km s ~1 from Deimos. To get off Phobos and drop down into the atmosphere of Mars for an aerobraked landing requires only 0.56 km s ~1 and a Mars landing from Deimos needs only 0.4 km s ~1. Thus return to a Space Station orbit about Earth from either of the Martian moons is easier than a comparable return from Earth’s Moon (assuming aerobraking in each case). The principal penalty for Phobos and Deimos return missions is the approximately 9-month travel time back to Earth.
The Near-Earth Asteroids
Asteroids as Threats and Opportunities. Recent developments in the astronomical search for near-Earth asteroids and in geological studies of impact features on Earth and other planets have presented us with a disturbing vision of the threat of disaster visited upon Earth by the impact of a near-Earth asteroid or comet. A technical overview of these issues can be found in the topic Hazards due to Comets and Asteroids (19) and in a popular discussion based on that volume (20). A quantitative treatment of impact hazards, based on the results of extensive and detailed Monte Carlo simulations of centuries of impact events on a populated Earth, has recently appeared (21). These studies show that, if a large asteroid, one massive enough to threaten human civilization, is now on a course to collide with Earth 100 years from now, then that body would almost certainly be one that we have not yet discovered: to date, we have found only about 10% of all kilometer-sized bodies that cross Earth’s orbit. These data also show that virtually all such large bodies can easily be discovered within the next few decades by a systematic, globally coordinated search and characterization program that costs less than a single small space mission. Using such a search program to give us adequate warning of a threatened asteroid impact, we would then have ample time to design, build, test, and deploy an effective defense against the threat.
That same search and characterization program, however, provides an exceptional opportunity. Many of the most dangerous asteroids have orbits that are remarkably accessible from Earth: they cross or graze Earth’s orbit about the Sun and can be reached, orbited, and landed upon more readily than any other body in the solar system. Fully a quarter of the near-Earth asteroids of all classes are easier to land on than the Moon: a given booster rocket could soft-land a larger payload on any of these than it could on the Moon. Further, these bodies have such feeble gravity that departure from them to return to Earth is vastly easier than departure from the Moon. Return of samples from the near-Earth asteroid Nereus, for example, requires a departure speed as low as 60 meters per second (135 mph), whereas departure from the Moon to return to Earth requires a speed of about 3000 meters per second. The amount of energy (or propellant) required per ton of returned material is 2500 times as large for lunar missions as it is for Nereus missions. For this reasons, exportation of bulk materials from the Moon to Earth could make sense only for fabulously valuable materials. Logistical Considerations. Because of the ease of returning asteroid-derived materials to Earth orbit, very large masses of materials may be moved. Logistical studies suggest that each ton of equipment launched from Earth to a near-Earth asteroid can return 100 tons of material to Earth orbit during the operational lifetime of the vehicle. Thus, assuming a launch cost of less than $500 per pound from the surface of Earth and using a 100:1 leverage factor, materials such as propellants or metals could be made available in Earth orbit for a few dollars a pound. The materials needed for future space transportation and construction would then be comparable in expense to those used in high-quality residential construction here on Earth.
In addition to these very favorable energy and logistical considerations, asteroids are attractive targets for a wholly different reason: they are rich in ”cheap” materials, such as water or steel, that are of great value and utility in space but outrageously expensive to launch from Earth. Further, the large majority of near-Earth asteroids contains high concentrations of extremely valuable precious and strategic metals, such as platinum, osmium, iridium, rhenium, and palladium, and semiconductor components such as germanium, gallium, arsenic, antimony, tellurium, and indium. The Earth-surface market value of this fraction is roughly $10,000 per pound, and the concentration of platinum, for example, is higher in the average NEA than in the best known terrestrial ore deposits. Experiments now underway in the laboratory of Prof. Henry Freiser at the University of Arizona, funded by a visionary private foundation that is interested in commercial development of space, are seeking simple, effective means to extract and separate many of these valuable resources from meteorites that are authentic samples of asteroids.
The idea of extracting asteroidal materials for commercial use is not new: As early as 1903, the great prerevolutionary Russian rocket visionary, Konstan-tin Tsiolkovskii, proposed exploiting asteroid resources. The father of practical rocketry, the American physicist Robert Goddard, wrote in a 1918 essay entitled The Ultimate Migration, of interstellar ships made from asteroids conveying our remote descendants away from the death throes of the Sun. The idea of mining asteroids was so visionary that Goddard sealed his manuscript away in an envelope labeled ”Special formulas for silvering mirrors,” where it languished unread for more than 60 years. Goddard’s reticence is understandable: the technologies required to carry out such ambitious schemes did not exist then, whereas critics and mockers incapable of understanding his ideas were legion. But today, this dream can be made a practical reality by applying the technology of the year 2000.
The keys to successful importation of materials from space are lower launch costs, careful choice of exploitation targets to favor those that are most accessible and have the richest resource concentrations, and minimizing the complexity of the operations to be undertaken by mining and extraction vehicles to rely on artificial intelligence, not human presence. The question how to lower the cost of access to space from about $5000 per pound to a few hundred dollars per pound is also an old one. Exactly a century ago, Tsiolkovskii wrote a science fiction novel in which the first successful manned venture into space was carried out in the year 2000… by a consortium of industrialists, scientists, and technologists funded by what can only be described as venture capital. Today, we see nearly two dozen companies, funded by venture capital and equipped with exciting ideas and the most modern aerospace and electronic technology, competing to lower the barriers in the way of massive development of space.
The NASA Near-Earth Asteroid Rendezvous mission, which arrived at the asteroid Eros in 2000 for a prolonged study of its surface, has already returned a wealth of data on the physical and chemical properties of that asteroid. Early data strongly suggest that the common S-type asteroids are chemically closely similar to ordinary chondritic meteorites, the most common class falling on Earth. X-ray and gamma-ray analytical experiments are planned to continue compositional mapping of Eros for many months.
The Space Development Corporation’s much-discussed Near-Earth Asteroid Prospector mission (NEAP) is merely the first example of attempts to privatize space activities. For planning purposes, it is assumed that NEAP is a mission to the asteroid Nereus. NEAP, although confined to a role as a science platform to fly NASA-funded instruments at low cost and to sell data gathered by privately funded instruments to NASA, can be regarded as an authentic prospector—a searcher for useful resources. The leading candidate instruments for the first mission are an alpha-proton X-ray spectrometer and multispectral imaging for global physicochemical mapping of the surface. The NEAP spacecraft bus can also accommodate several small experiments, such as a rover, that could be soft-landed on the surface of the asteroid after the primary mapping mission is complete. A second step would be to land processing experiments on the surface of an asteroid and demonstrate small-scale production of water or metals. Because return of materials from so many NEAs is exceptionally easy, both virgin surface samples and processed materials may be automatically returned to Earth more easily than samples could be returned from the Moon (a technique the Soviet Union that demonstrated successfully on the unmanned Luna 16, 20, and 24 missions in the 1970s).
Given the accessibility of NEAs and the diversity of their resources, some idea of the amount of available resources is in order. Perhaps the first example of note is the metallic near-Earth asteroid called Amun. This asteroid is about 2000 meters in diameter. If it were to strike Earth, it would deliver a devastating blow of 10 million megatons (10 teratons) of TNT, several thousand times the explosive power of a nuclear world war. Amun, the smallest known metallic asteroid of the several dozen known, contains several times as much metal as the entire amount of metals mined and processed during the history of humankind. A conservative estimate of the market value of this asteroid is $5 trillion. Resource Richness and Diversity. The entire NEA population, which has very diverse chemical and physical properties, contains vastly more material than Amun. An estimate of the overall composition of the NEA population is shown in Table 4. It is not difficult to estimate how much of each of a wide variety of commodities, such as water, carbon, nitrogen, metals, and phosphorus, is required in circulation to maintain one average human at present-day North American, Western European, or Japanese levels of affluence. From these figures, we may estimate how many people could be supported indefinitely by the resource wealth of the NEA population, assuming a fully recycling regime powered by the Sun. According to Table 4, the number is probably close to 14 billion people. Nitrogen, principally in its role as a fire-suppressing diluent of atmospheric oxygen, appears to be the limiting resource. One of the most remarkable lessons of Table 4 is that the proportions of materials needed by civilized human beings is similar to the proportions in asteroids.
But the NEAs are only a small part of the picture. Most NEAs follow orbits that take them out to the heart of the Asteroid Belt, between the orbits of Mars and Jupiter, at aphelion. Thus a processing unit landed on a typical NEA will get a free round trip to the Belt and back on each trip around the Sun (typically, once every 3-5 years).
Table 4. Resources of the Near-Earth Asteroids3
| Commodity | Mass among NEAs, | Per capita inventory, | Population sustainable |
| 1015g | g/person | by NEA resources, billion people | |
| Silicate | 2500 | 140,000,000 | 17.8 |
| Ferrous metals | 300 | 20,000,000 | 30.0 |
| Fe in oxides | 300 | 6.0b | |
| Cement | 60 | 10,000,000 | |
| Industrial CaO | 2,000,000 | 30.0 | |
| Phosphates | 10 | 2,000,000 | 5.0c |
| Water | 300 | 10,000,000 | 30.0 |
| Carbon | 100 | 1,000,000 | 100.0d |
| Nitrogen | 10 | 700,000 | 14.0e |
| Sulfur | 60 | 1,200,000 | 50.0 |
| Sulfides | 150 | 1,200,000 | 125.0 |
In the likely order of development, water is the first asteroidal resource worthy of attention. Its uses as a propellant and as a life-support material are obvious. Second in order would be native ferrous metal alloys, whose major components, iron and nickel (and possibly cobalt), would be retained in space for constructing space-based facilities such as solar power satellites. The rare and very valuable precious metals and semiconductors in asteroidal metal alloys are worth returning to Earth. Dr. Jeffrey S. Kargel has explored the effects of large-scale importation of these materials from space on the market size and prices of these commodities on Earth. He concludes that prices will decline less rapidly than the rate of supply increase because of new uses stimulated by lower prices.
The potential customers for the materials that remain in space include government agencies that need propellants for injection into geosynchronous orbit, for orbital stationkeeping, or for departure from Earth’s gravity well, such as both unmanned and manned Mars missions. In addition, civil traffic bound for geosynchronous orbit would be an important market for propellants. Metals would be of use for constructing and shielding large structures, of which the most obvious commercial example would be Solar Power Satellites. But this entire scenario depends on a stable and rational legal and regulatory system in which investors will have reasonable assurance that the fruits of their ingenuity and investment will not be arbitrarily obstructed, or even confiscated. We return to this issue later.
Proposed System Architectures. There are two central questions regarding the exploitation of asteroidal resources. First, there are the propulsive energy constraints imposed by the geometry of the orbit of the target asteroid. Outbound DV requirements from reference orbits such as LEO (a circular orbit at 300-600 km altitude, compatible with future space stations) have been calculated for almost every known near-Earth asteroid. In general, the typical NEA of interest has a perihelion close to or within Earth’s orbit. Many NEAs have aphelia in the asteroid belt. The best candidates have low orbital inclination, perihelia (or, in the case of Atens, aphelia) very close to 1.00 AU, and low eccentricities. Outbound DVs (from LEO to landing on the target asteroid) as low as 3.2 km/s are allowed by theory, but in practice bodies whose outbound DVs are less than about 4.2 km/s are so vulnerable to perturbations and capture by Earth that their populations are depleted. About 15% of all NEAs have outbound DVs that are less than the DVrequirement for missions from LEO to a soft landing on the Moon (6 km/s). Return from many NEOs requires DVs less than 1km/s; several lie below 0.4 km/s. The best known return DV is 0.06 km/s from Nereus (1982 DB). By comparison, return to Earth intercept from the lunar surface requires roughly 4 km/s, depending on where the launch site is on the Moon.
Second, there is the choice of location for the space base in near-Earth space from which missions depart and to which they return with their cargoes. In a study of opportunities for missions to the Mars system, Benton Clark argued that the most suitable base for long-term, round-trip use would be in highly eccentric Earth orbit. A typical base orbit might have a perigee of several thousand kilometers, beyond the heart of the Van Allen radiation belts, and an apogee of 40,000 to as much as 400,000 km (roughly the orbit of the Moon). Both aero-braking and propulsive capture require that the hyperbolic excess velocity be lost as close to Earth as possible and the perigee be about 100 km. A small apogee burn can then lift the perigee safely out of the atmosphere. From such an orbit, the velocity required for escape from Earth is extremely modest, and from such an orbit, it is easy, by a brief apogee burn, to drop the perigee down to the top of the atmosphere either for return to Earth or for an engine burn to escape Earth at maximum efficiency.
Solar Power from Space
In recent years, debate concerning the greenhouse effect, global warming, fossil fuel burning, and the environmental impact of mining and transporting fossil fuels has focused increasing attention on alternative sources of electrical power. The desire is for cheap, clean, abundant future energy supplies motivates consideration of solar power.
There are several ways in which solar power may be made available on Earth. The first and most obvious option is constructing solar voltaic cell “farms” in locations on Earth’s surface where the total insulation is highest. Earth-surface solar cell arrays are subject to the day-night cycle; atmospheric attenuation of sunlight by Rayleigh scattering and by cloud opacity; shadowing by windblown dust (an especially common phenomenon in areas that have the most reliable solar illumination); or by smoke, haze, or volcanic aerosols, and damage by wind, snow and ice loads, hail, lightning, and flying objects.
An obvious alternative is to locate the solar cell arrays in space, where performance (watts of electrical power per square meter of collector area per year) can be 2 to 10 times higher than on Earth’s surface (22). The principal drawbacks of space-based solar collectors are the initial cost of launch and the necessity of beaming the power down to receiving antennae on Earth as microwave power. The choice of the orbit of the solar cell array has an important influence on system performance. The easiest orbits to achieve (low Earth orbit; LEO) usually have a 50% duty cycle due to passage through Earth’s shadow and also traverse the 300 to 1000-kilometer altitude range where the probability of collision with orbital debris is high. Higher orbits have greater installation costs and less opportunity for service but better exposure to the Sun and a more favorable debris environment. Most point designs of Solar Power satellites (SPS) assume that they will be installed in geosynchronous orbit. Large-scale reliance on SPS constellations would entail installing approximately 5x104km2 of solar collectors in the geosynchronous belt, where they would far outshine the brightest stars.
An interesting variant of the SPS concept that would avoid the light pollution problem is David R. Criswell’s scheme of building solar-power collectors on the Moon, using lunar resources for collector construction (23). This approach saves the cost of lifting most of the mass of the SPS system from the Earth.
SPS constellations in Earth orbit could also benefit greatly from using nonterrestrial materials. A recent NASA study (24) showed that SPS are close to being economically viable, even with Earth launch (“uphill” transport) of all of their components. Any scheme that derives the most massive, low-tech SPS components from nonterrestrial metals (brought “downhill” to Earth orbit) holds promise of making them highly competitive economically.
Some words are also in order regarding the environmental impact of space resource use. In general, any industrial activity that can be off-loaded from Earth eases the environmental burden on Earth’s biosphere. An enormous proportion of Earth’s environmental troubles are related to mining, refining, transporting, and using fossil fuels. National policies aimed at reliance on solar power satellites mitigate all of these problems, simultaneously making the United States the world’s largest exporter of energy and energy technology and extending the usefulness of our limited petroleum reserves far into the future by dedicating crude oil to petrochemical production, not combustion. Finally, such an approach reduces or eliminates American dependence on foreign sources of crude oil.
Long-Term Prospects
For logistical and programmatic reasons, the most attractive locations for resource extraction are the near-Earth asteroids, the Moon, and the Mars system, including Phobos and Deimos. But, in the longer term, other options may be considered. As mentioned earlier, Mercury’s polar ice deposits provide a potential source of propellants for a sample return mission. Venus, by reason of its enormous atmospheric pressure (92 atmospheres), scorching surface temperatures (750 K, or about 900°F), highly corrosive atmosphere (containing gaseous hydrochloric and hydrofluoric acids and clouds of sulfuric acid droplets), and high escape velocity (10 kilometers per second), is among the most unattractive locations in the solar system, similar to Dante’s vision of Hell.
Near-Earth asteroids typically reach the heart of the Asteroid Belt at aphelion. Any equipment riding on an NEA is transported out to the main Belt every few years. It is easy to envision a device riding on a water-bearing NEO out to the Belt, manufacturing propellants from the water as it goes, and using that water to transfer from the NEO to a convenient Belt asteroid. Such a transfer is energetically no more difficult than returning to Earth. Emplacing equipment on Belt asteroids in this manner is logistically more complex than landing on an NEO but not more demanding from a propulsive or launch-weight perspective. In effect, systematic exploitation of the NEO population makes access to the main Belt easy.
The mineral riches of the Asteroid Belt are almost beyond comprehension (Table 5). The asteroids that reside in the Belt make up a very large population, roughly 40,000 bodies larger than a kilometer in diameter, and the total mass is roughly a million times as large as the total mass of the NEA population at any one time. Table 5 summarizes the total amount of resources available in the Belt in a manner similar to the NEAs treated in Table 4. The conclusions are staggering: the materials available in the Asteroid Belt would maintain indefinitely a human population of at least 10,000,000 billion people—about one million times the maximum carrying capacity of Earth. Assertions that we are running out of resources reckon without twentieth and twenty-first century technology. The supply of resources available to a spacefaring humanity is effectively infinite. But there is no possibility of returning all that material to Earth (enough steel in the Asteroid Belt, for example, to build a steel-frame building 8,000 stories tall covering all the land area of Earth), nor is there any possibility of accommodating so many people on one planet. The bulk of these resources would be used in space.
Table 5. Resources of the Asteroids Belta
| Commodity | Mass in the belt, | Per capita inventory, | Population sustainable |
| 1021g | g/person | by belt resources, billion people | |
| Silicates | 2500 | 140,000,000 | 17,800,000 |
| Ferrous metals | 300 | 20,000,000 | 30,000,000 |
| Fe in oxides | 300 | ||
| Cement | 60 | 10,000,000 | 6,000,000b |
| Industrial CaO | 2,000,000 | 30,000,000 | |
| Phosphates | 10 | 2,000,000 | 5,000,000c |
| Water | 300 | 10,000,000 | 30,000,000 |
| Carbon | 100 | 1,000,000 | 100,000,000d |
| Nitrogen | 10 | 700,000 | 14,000,000e |
| Sulfur | 60 | 1,200,000 | 50,000,000 |
| Sulfides | 150 | 1,200,000 | 125,000,000 |
Beyond the Belt, in two extensive clusters located on the orbit of Jupiter, 60° ahead of and behind Jupiter, are the Trojan Asteroids. The total mass of material in these two vast clouds is probably several times the total mass of the Belt. At the same distance from Earth are the satellites of Jupiter. The largest four, the Galilean satellites Io, Europa, Ganymede, and Callisto, lie so deep in Jupiter’s gravity well and so far inside its intense radiation belts that they are demanding destinations for even flyby spacecraft. The four small inner satellites are in an environment that is essentially not survivable. The two families of small outer satellites, however, are dynamically and probably even chemically indistinguishable from the Trojan asteroids. Saturn also has several small, remote satellites. But the great distance of these bodies from any plausible site of demand for their resources and the very low light levels at such great distances from the Sun (27 times less at Jupiter than at Earth) argue against any short-term economic significance.
The gas-giant outer planets, Jupiter, Saturn, Uranus, and Neptune, offer astronomical quantities of attractive resources situated at the bottom of very deep gravity wells. The easiest of the giant planets to land on and return from, Uranus, is at the very limit of the capability of single-stage nuclear rockets. But if high-performance liquid- or gas-core nuclear rocket engines, or fusion rockets, can be built, then the helium-3 fusion fuel resources of their atmospheres would become accessible to Earth. Uranus alone contains enough helium-3 to support a population of 10 billion people at present-day North American or European consumption levels for more than 1015 years, or to support a million times as many people from now until the Sun dies of exhausting its hydrogen fuel.
Legal and Treaty Issues Governing Space Resource use
It is not sufficient merely to summarize the scientific data on the nature and orbital properties of potential space resources and outline proposed extraction, processing, fabrication, and transportation schemes attendant on their use. It is also necessary to survey the legal issues involving claims of ownership, national sovereignty, and mineral claim registration and certification. Present Legal Regime. The booklet Agreement Governing the Activities of States on the Moon and Other Celestial Bodies contains the texts and history of a number of international treaties and agreements regarding space resources (25). The original agreement, and hence the basis for all more recent treaties, was laid by the 1967 Treaty on Principles Governing the Activities of States in the Exploration and Use of Outer Space, Including the Moon and Other Celestial Bodies, usually referred to as the Outer Space Treaty. Article I states that
The exploration and use of outer space, including the moon and other celestial bodies, shall be carried out in the interests of all countries, irrespective of their degree of economic or scientific development, and shall be the province of all mankind.
It further states that the exploration and use of celestial bodies shall be done ”without discrimination of any kind.” Article II proclaims, ”Outer space, including the moon and other celestial bodies, is not subject to national appropriation by claim of sovereignty, by means of use or occupation, or by any other means.” Article VI binds each signatory State to take responsibility for ”national activities in outer space, including the moon and other celestial bodies, whether such activities are carried on by governmental agencies or by non-governmental entities…” Article VII assigns liability for damage by space missions to the originating State, a statement of the principle embodied in the 1972 Convention on International Liability for Damage Caused by Space Objects. Article XII requires that all ”stations, installations, equipment and space vehicles on the moon and other celestial bodies shall be open to representatives of the other States Parties to the Treaty” and to other States on the basis of reciprocity. Article XIII places intergovernmental agencies under the same Treaty obligations as individual States.
In general, the Outer Space Treaty assumes permanent domination of space activities by governments, a phenomenon that accurately characterized the 1960s, and further assumes that commercial ventures could be dismissed without individual consideration by simply assigning them to the nearest available government. Private property is not even mentioned. The 1967 Treaty was ratified by every space faring nation and reflects a very broad international consensus. For our purposes, the most important single feature of the Outer Space Treaty is the assertion in Article I that uses of space are ”the province of all mankind.” The interpretation of this phrase is almost infinitely varied, reflecting the political view of the interpreter. The most basic interpretation is that everyone has the right to participate in exploration and use of space and no one can be denied that right; the most ambitious would require representation of (or permission from) every nation on every mission. Acceptance of the Treaty by a wide range of nations that have different and often contradictory political and economic ideologies suggests that agreement on its wording simply masks disagreement on its meaning and application.
The most relevant document concerning exploitation of asteroids is the 1979 Agreement Governing the Activities of States on the Moon and Other Celestial Bodies, usually known as the Moon Treaty. Draft materials for this treaty and an account of the negotiations that led to it are included in a U.S. Government Printing Office publication, 59-896 O (25). The final agreement, adopted and opened for ratification by States by General Assembly Resolution A/RES/34/ 68 bearing the same name, consists of 21 articles.
Article 1 states that all other celestial bodies and the Moon are covered by this agreement, excepting meteorites that fall to Earth “by natural means.” Article 7 prohibits ”the disruption of the existing balance of the environment” and calls on States to avoid ”harmful contamination” of the body as well as ”harmfully affecting the environment of Earth.” Article 8 guarantees the right to land, establish bases, and travel over the surfaces of these bodies. Article 9 enjoins these States to ”use only that area that is required for the needs of the station.”
Article 11, by far the most important for our purposes, states ”the Moon and its natural resources are the common heritage of mankind.” Further, ”the moon is not subject to national appropriation by any claim of sovereignty, by means of use or occupation, or by any other means.” Neither the surface, subsurface, nor natural resources of the Moon ”shall become property of any State, international governmental or non-governmental organization, national organization or nongovernmental entity or of any natural person.” Installation of equipment or bases does not create any claim of ownership.
The States Parties to this agreement hereby undertake to establish an international regime, including appropriate procedures, to govern the exploitation of the natural resources of the moon as such exploitation is about to become feasible.
States Parties are required to report any natural resources discovered on any celestial body to the Secretary-General of the UN. Of course, in reality, the astronomers who examine the spectra of asteroids and find that they are made of metals or hydrated minerals are completely unaware of this treaty obligation and of the possible role of these materials as resources. Therefore, none of the roughly 1000 spectrally characterized asteroids has ever been ”reported” to the Secretary-General. In closing, Article 11 proclaims that
The main purpose of the international regime to be established shall include: a) the orderly and safe development of the natural resources of the moon, b) the rational management of those resources, c) the expansion of opportunities in the use of those resources, and d) an equitable sharing by all States Parties in the benefits derived from those resources, whereby the interests and needs of the developing countries, as well as the efforts of those countries which have contributed either directly or indirectly to the exploration of the moon, shall be given special consideration.
Article 12 allows ownership of devices and stations placed on the Moon, and Article 14 places full responsibility for all activities on the Moon upon the State from which that activity originates. Here again, the possibility that commercial entities will operate in space is simply ignored. Article 15 requires that every installation on the Moon be open to inspection visits from any other State, and the remaining Articles refer to the role of international governmental organizations and to the ratification and amendment of the Agreement.
It is clear that the nations that contributed to this document accept absolute centralized control, common ownership of these resources, and required “sharing” (confiscation) of much of the proceeds of the work. The treaty seems to offer nothing for any State that can conduct operations on the Moon.
The United States and essentially every other nation that has space faring capabilities are signatories to all of the other treaties and agreements cited; however, with almost equal unanimity, these same nations have failed to ratify the Moon Treaty. Many other nations have ratified it, hoping perhaps for windfall profits from the labor, investment, and invention of others. Suggested Legislative Actions to Support Space Development. Perhaps it would be more fruitful to ask what legal, regulatory, and economic regime would permit development of space resources. Which matters should be subject to international regulation, which functions should be carried by national governments, and which should be left to the free will and choice of the private and public space faring parties themselves? It appears that the appropriate role of government should be defined. We must acknowledge that opening the space frontier, like opening the American West, can be assisted or hindered by governmental actions. Just as in the establishment of railroads in the West, government assistance, not domination, is useful in several areas. The first four areas are of general applicability to space development:
1. Governments can support private space endeavors by buying scientific data, in effect, privatizing many research missions. The Near-Earth Asteroid Prospector (NEAP) mission is but the first example of this approach and offers a cost structure that reflects competitive commercial practice rather than the inefficiency that is characteristic of monopolies. NEAP and its likely near-future competitors will offer prices about a factor of4 lower than the cost of doing a comparable mission from within the government. This improvement is above and beyond the already substantial cost reductions brought about by NASA under the administration of Mr. Goldin. This goal will largely be achieved in the United States under the provisions of the Commercial Space Act of 1997.
2. Governments should support the development of low-cost space transportation systems by buying launch services competitively from private vendors. A good start on this initiative was made in the United States by the Launch Services Purchase Act of 1990.
3. Governments, through NASA, ESA, Glavkosmos, or equivalent agencies, should take a leading role in developing key electronics technologies to assist early economic development of space, as in the early days of experimentation with communication, navigation, earth resources, and weather satellites.
4. Governments should play a major role in developing critical propulsion technologies. At a bare minimum, solar thermal and solar sail propulsion systems should be developed and placed in the public domain. The Russian test of the Znamya steerable solar mirror in space in November 1998 was relevant to this capability.
Three more goals are strongly specific to space resource development:
5. Governments should take the lead in developing technologies for extracting and processing the most important mineral resources. The technologies that should be tested in microgravity at the International Space Station, or elsewhere, include crushing and magnetic separation for extracting of native ferrous metal alloys and extracting water from ice lenses, permafrost, hydrated salts, and clay minerals. Initially, water may be used directly in solar thermal or nuclear thermal rockets, but it is clear that, in the longer term, converting water into cryogenic propellants will be highly desirable. Electrolysis of water into hydrogen and oxygen and liquefaction of both gases to make liquid rocket propellants must also be adapted to operation in very low (or artificial) gravity. Many of these technologies are extensions or adaptations offamiliar Earth-surface processing technology to high-vacuum, microgravity environments. Metals mining and processing concerns have extensive Earth-related experience but require expert assistance from universities and government research centers in selecting their targets and from the government and the aerospace industry in adapting their experience to space operations. Given the present precarious financial state of the aerospace industry, its participation in the experimental stage of such an endeavor would almost certainly require government support. As recently as 1993, NASA was spending more than $2.5 million per year on research into the use of nonterrestrial resources. This endeavor has been heartily endorsed by the NASA Administrator in many public addresses, but nonetheless, funding has essentially disappeared.
6. Governments should take the lead in purchasing products produced in space for use in space. The leading example is rocket propellants: the costs of ambitious deep-space missions are raised enormously by the cost of lifting the required fuel load for the outbound trip out of Earth’s gravity well— at a cost of several thousand dollars per pint. Instead, all outbound missions that pass through low Earth orbit could be refueled with high-performance asteroid-derived liquid hydrogen and liquid oxygen from a ”gas station” in low Earth orbit, perhaps an adjunct to the Space Station. Because the government would simply purchase high-performance, cheap, space-derived propellants that are competitive with Earth-launched pro-pellants, this activity would also save the taxpayer money. Up-front investment in experimenting with the transfer of cryogenic propellants in microgravity would be required before any benefits could be realized. It is interesting that the Soviet Union has used in-orbit propellant transfer routinely and without incident since the 1970s, whereas the United States has never developed the capability. Russian experience would be very useful in this area. Beyond propellant manufacture, the most promising single space resource for immediate exploitation is native ferrous metals, which could be used for large-scale space construction. Using asteroidal metals to build Solar Power satellites appears to be the easiest route to energy self-sufficiency for the United States, Japan, and Europe. 7. Finally, in the light of the economic potential of the points discussed before, governments must be prepared to deal with certifying private claims of ownership and mineral claims on bodies in space. Each national government could establish a formal means of registering these claims that will assure entrepreneurs that the integrity of their claims will be recognized, a necessary precondition to raising large amounts of venture capital. Governments interested in space development will predictably avoid international legal entanglements such as the original Law of the Sea treaty that have a confiscatory attitude toward profit and new technologies. Such treaties prevent the development of resources that would ease the lot of all humankind. Developing nations must understand that opening up new resources and reducing costs is to their direct benefit, that they too may directly participate in such activities. As pointed out earlier, the resources available in nearby space are so large and the cost of access to space will soon be so low, that the idea of domination by any monopoly is absurd. The most important effect governments can have on space development is to lay a firm foundation for commercial, competitive, private development of space resources.
Possible International Regimes. For the purposes of the following discussion, we will assume that some profitable form of space-resource enterprise exists or demonstrably could exist. If this were not true, none of the preceding questions would be of any interest. “Profitable” means that the proposed space activity can either provide a commodity in some economically meaningful location at lower cost than its competition, provide much larger amounts of that commodity at comparable prices, or provide a new commodity not already on the market. In all of these cases, it is to the economic advantage of the buyer, the entrepreneur, and the governmental entities that tax profits to allow such an activity to flourish. This is true even without allowing for the environmental advantages of relegating energy and mineral mining to space, which positively impact every person on Earth. It is perfectly true that certain existing interests, especially those that have monopolies on rare materials, would be harmed by such competition based on new resource bodies and new technologies. But this has always been true and always will be. Successful mining concerns are those that have taken the lead in adopting new technologies and developing new ore bodies. Metals concerns such as INCO will face the choice of trying to compete with space-derived resources or of taking a leadership role in their development. All consumers will benefit; only those corporations ready and willing to adapt will benefit from this new trillion-dollar market.
Therefore, we require that some means be instituted to make space mining possible, on the grounds that it is advantageous to humanity. This in turn requires that the entities that mine, extract, and fabricate space resources be given a regulatory regime in which investments would be rational. There are enough economic and physical risks associated with space mining that adding the risk of an unstable or politicized regulatory environment would be fatal. Therefore, it is essential to register, recognize, and enforce mining claims.
The simplest, and in many ways most attractive, scheme would have the United Nations serve as a registry for mineral claims, much in the same way that it maintains a registry of launchings of space vehicles without exercising any control or authority over the activity. The World Court could also provide a venue for claim registry. Another method, inspired by patent law, would allow private individuals or corporations of any nation to register mining claims with their own government, with full mutual recognition of claims. In practice, nations could enter this arena one or two at a time, executing reciprocal agreements with the other relevant governments, as the need arises. Adjudication of conflicts might fall within the venue of the World Court or national courts. The World Court’s role is limited by the historical absence of any enforcement ability. Enforcement would, of course, be carried out on Earth: the science-fiction device of asteroid miners declaring autonomy is not a near-term option, so long as space activities are highly dependent on Earth for both equipment and markets.
Several levels of presence might serve as the threshold for making a valid mining claim:
1. at the lowest level, discovery of an asteroid;
2. remote spectral characterization of an asteroid demonstrating the presence of an economically attractive resource (i.e., an ore);
3. physical presence of an unmanned vehicle to document the presence and setting of an ore;
4. physical presence of an unmanned vehicle, documented by sample return to Earth;
5. physical presence of a human crew that proclaims mineral rights or ownership; and
6. presence of an established human settlement.
It is noteworthy that, of the roughly 15000 assteroids discovered and cataloged to date and of the nearly 1000 that have been subjected to photometric or spectroscopic study, not one single astronomer has ever registered a public claim of mineral rights or of ownership. This is prima facie evidence for a universal consensus that such data do not constitute valid grounds for a claim. Maritime law has provided a precedent for claiming ”mining” rights to shipwrecks through reconnaissance by unmanned vehicles that visit and document the ”ore.” Some space law theorists have suggested awarding a higher level of claim to those who return a sample to Earth. However, there seems to be no clear legal precedent for this requirement, and a host of scientific and engineering reasons to suppose that physicochemical characterization by spacecraft instrumentation provides all of the essential information that a returned sample could provide. The need to assay for the abundance of a particular ore, an economic necessity in mineral claim assessment on Earth, is largely irrelevant on homogeneous asteroids.
For this reason and others, there seems no need to require human presence because the evidence needed to establish the presence of an ore can readily be acquired without human presence. Further, requiring human presence places an enormous economic barrier in the way of asteroid resource exploitation and effectively prevents private corporations and small nations from participating. Only a handful of superpowers could afford to carry out such a mission, artificially turning space resource exploitation into a monopoly or elitist activity. This is precisely the opposite of the desirable regime, in which many nations and companies can participate and in which free competition places constant pressure on prices, to the benefit of consumers. Note that manned presence on another celestial body, the Moon, has not resulted in any claim because the entity that carried out the Apollo project was NASA, a government agency that, by recognized international law, cannot make a claim of national sovereignty. The suggestion that the United States Congress should pass a law authorizing an ”extraterrestrial land claim made by any private entity that has established a true space settlement” is completely inadequate. This is equivalent to a home builder not being allowed to seek ownership of the land on which the house was built until after he had built his house and moved in. In a commercial regime, investors need reasonable prior assurance that their capital is not being squandered. Claim recognition must precede development.
In general, the vast amount of resources available among the near-Earth asteroids and in the Asteroid Belt suggests that competition for mining claims should not be a common problem. Nonetheless, for any particular resource such as water, there may be a single asteroid that is significantly more accessible than others. There would then be a strong incentive to get there first and file a mineral claim that included the entire asteroid. For a 100-meter or 1000-meter body, comparable in size to an open-pit mine on Earth, it would be perfectly reasonable for the first arrival to claim the entire body. On the Moon, filing a mineral claim for, say, the entire Mare Imbrium, would be an absurdity, comparable to claiming all of Colorado or Switzerland as a mine site. Further, on a very small asteroid, minor environmental disturbances caused by one mine (dust, for example) might materially hinder other activities on the same body. Although it is true that the asteroidal environment is rapidly self-cleaning (through reaccretion of dust and Poynting-Robertson removal of escaped dust), the short-term local effects on visibility may be serious. A reasonable criterion might be to allow claims of all of the material of that body within 1 kilometer of the designated mine site in all directions, excepting any material already claimed in conformance with this criterion. Therefore, documentation of the claim must include physicochemical data on the surface and also a good three-dimensional map of the body where the mine site is specified. An actual landing at the mine site (not necessarily permanent occupancy) may be mandated, although there seems to be no convincing reason to require it. The concept of an ore body has quite a different meaning on an asteroid from usual terrestrial experience. Except for composite asteroids assembled by chance low-velocity collisions, most NEAs should be compositionally uniform. The physical state (dust, sand, cobble, boulder, country rock) of the resource is usually a more important determinant of mine-site location than the composition, which will usually be very uniform. There will rarely be veins of ore to follow. Further, the dross from extracting any resource (such as water from a carbonaceous asteroid) will itself be a very valuable resource, containing an abundance of other volatiles and both ferrous and precious metals.
This brings us to the issue of actual private or corporate ownership. A valid claim confers essentially all of the benefits of ownership except, perhaps, the ability to sell or license the claim. This author would regard a claim that can be sold as the moral equivalent of property. Allowing claims to be sold permits optimizing expertise in prospecting, site-study, and mine-development specialists. Mineral claims that carry the right of sale or licensing would be completely satisfactory.
It must be emphasized that no entity that has met the basic requirements of either human presence (NASA astronauts on the Moon), or in situ representation by an unmanned spacecraft (American Viking and Pathfinder and Soviet Mars Landers on the Martian surface), has ever claimed property rights or national sovereignty. Certainly, those individuals who presently purport to be selling tracts of land on the Moon and Mars have no basis whatsoever for claiming ownership in the first place. They lack both the right to sell what is not theirs and the legal or juridical authority to act as registrars of ownership. The existence of such schemes serves to discredit legitimate mineral rights or ownership claims based on reasonable criteria of presence. Similarly, the existence of ad hoc registries for claims, such as that of Professor Lawrence D. Roberts of the Archimedes Institute, can be viewed in the same light as the proceedings of a moot court. The existence of this registry may serve to stimulate awareness of the problem, so that some official mechanism for claim registry can be brought into existence. The issue of claim registration is made timely and urgent by the impending Near-Earth Asteroid Prospector (NEAP) mission of the Space Development Corporation. The time for initiating a recognized claim registry system has arrived.