Introduction
Attempting to predict human responses to space travel, physicians and researchers of the 1950s speculated that microgravity and spaceflight itself would present significant challenges-if not barriers—to the human body (1,6). They hypothesized that the combined stresses of launch acceleration, weightlessness, radiation, and heavy deceleration upon reentry would be incapacitating. At the very least, they predicted that the bodily systems sensitive to gravity-based cues would function improperly or not at all. Given this grim forecast, the initial focus was to demonstrate that life, away from Earth, could survive space travel and subsequent return to Earth’s gravity. Faced with this challenge, both the United States and Soviet Union turned first to ground simulations, such as immobilization studies, and then to the study of animal test subjects launched on board high-altitude balloons, suborbital, and orbital rockets. What followed is a series of biological satellites that carried a variety of living specimens, from isolated cell cultures to whole instrumented organisms. The Soviet Union relied primarily on canines to provide such data, whereas the United States chose primates for such experimentation.
There is not much doubt that in the beginning of the U.S. space exploration effort, the primary motivation was very real competition with the Soviets. What is remarkable is that in 1969, at the very height of this competition, when the
Americans first landed on the Moon, a long-lasting collaborative program in space biology was initiated by the United States and the Soviet Union. In that year, NASA flew the U.S. built “Biosatellite”. This was a complex satellite that was placed in near-earth orbit with an appropriately instrumented chimpanzee as the payload. “Biosatellite” was flown and was successfully recovered after a week in orbit. Numerous measurements were made, but ultimately NASA rated the mission as only ”partially successful,” because of the death of the primate early in the post flight period, due to complications from loss of fluids and potentially an infection, thus loss of valuable data.
The ”Biosatellite” mission was managed by the NASA-Ames Research Center because Ames was NASA’s lead center in space biology. With the Apollo program in full gear, NASA decided to cancel all planned biological flight programs, except for a small experiment involving the behavior of a frog’s otolith in zero gravity. The response from the leadership at Ames was to seek permission from NASA Headquarters to initiate collaboration with the Soviets in this area. Drs. Harold P. Klein and Joseph C. Sharp succeeded in making the case, and they received permission to go ahead. Eventually, several distinguished Soviet scientists were invited to visit Ames. This group included Professor Alexander Oparin, who was a distinguished expert in exobiology, and Academician Oleg Gazenko, an important leader in the Soviet space organization.
Eventually, an agreement was reached that would call for U.S. scientists to develop biological payloads to be flown on Soviet “Vostok” spacecraft. Dr. Sharp was the coordinator on the U.S. side, and Dr. Eugene Ilyiin, Dr. Gazenko’s deputy for biology, was the Soviet leader of the program. The first payload was flown in 1975. Since then, there has been an average of one flight every 2 years, and some significant scientific results were obtained early in the program (2,4). The early success of this program led to a sustained cooperative effort that survived several U.S. – Soviet confrontations during the Cold War. The Soviet invasion of Afghanistan in late 1979 caused President Carter to terminate many relationships that had been established as the Cold War wound down. For example, U.S. participation in the Moscow Olympic Games in 1980 was canceled. However, early in 1980, an American biological payload was flown aboard a Soviet satellite. Appeals from the scientists involved in this work prevailed to keep things going.
The success of the U.S. - Soviet collaboration, which also included exchange of biomedical data from piloted missions, was followed by the much higher profile 1975 Apollo. Soyuz Test Project Joint U.S. – Soviet flight and docking of the respective countries, spacecraft in low Earth orbit. This program was the result of a diplomatic effort during the Nixon – Ford Administrations. To promote a ”detente” in the Cold War, it was felt that a joint U.S. – Soviet space mission would be helpful. The joint U.S. – Soviet crew performed a number of scientific experiments, but it was clear that the primary justification was symbolic. In that sense, the linkup of the U.S. Apollo and the Soviet Soyuz spacecrafts, which led to the famous ”handshake in space,” was a successful effort that aided the process of ”detente” (3).
Serious thinking about possible scientific U.S. - Soviet collaboration of people in space was triggered by the successful first flight of the new Space Shuttle ”Columbia” in April 1981. The Soviets had deployed the ”Salyut 7” space station in 1982, and the existence of these capabilities once again led to the development of some specific proposals for collaborative efforts. In 1982 and 1983, Air Force Lieutenant General James A. Abrahamson, who was serving as NASA’s Associate Administrator for Space Flight at the time, proposed a ”people exchange” between ”Columbia” and ”Salyut 7.” The idea would be to have ”Columbia” fly close to the ”Salyut 7” station. An Astronaut from ”Columbia” would perform an extravehicular activity (EVA), do a ”space walk,” and enter ”Salyut 7.” This would be followed by a Soviet Cosmonaut leaving ”Salyut 7” and joining the crew of ”Columbia.” A preliminary proposal was developed but was not mplemented for various technical and political reasons that existed at the time.
The end of the Cold War in 1991 led to an expansion of U.S. - Russian collaborative activities in space. A series of meetings between the U.S. Vice President, Albert V. Gore, and Russian Prime Minister Victor Chernomyrdin resulted in agreements that involved the collaborative use of the Russian ”Mir” Space Station, as the Russian participation in and contribution to the International Space Station Program. James Beggs and, the NASA Administrator and Deputy Administrator, secured Administration and Congressional support to initiate the latter facility in 1984. From the very beginning, the U.S.-led space station program was intended as an international effort. The European, Canadian, and Japanese Space Agencies had strong roles in the beginning, and in 1993, Russia was brought into the program. The Russian contribution was to build various segments of the Space Station and to use Russian ”Proton” launch vehicles to resupply the Space Station. The Russians also agreed to provide the Soyuz TM spacecraft as an initial crew rescue vehicle. The NASA Administrator Daniel S. Goldin initiated the International Space Station redesign effort to accommodate all of the international partners, improve research capacity, and provide long-duration spaceflight training for U.S. astronauts and ground personnel. It was also a rehearsal for international partners of living and working together in space. In addition, NASA resources were used for more than 300 Russian space scientists for competitively selected research projects and collaboration with their U.S. counterparts. This effort provided much needed resources to the Russian space science community at the time of economic transition in the post-Soviet era. The Research program on the MIR Space Station was developed by Drs. Arnauld Nicogossian and Carolyn Huntoon for NASA. Dr. Nicogossian was responsible for overseeing the implementation of the research program on ”MIR” and the funding of the Russian space science community.
These efforts began in 1995 with a rendezvous in space between the Space Shuttle ”Discovery” and the ”Mir” Space Station. An astronaut-cosmonaut exchange was executed later in 1995 in a docking of Space Shuttle “Atlantis” and ”Mir” that finally fulfilled what General Abrahamson had proposed more than a decade earlier. The difference between what was done in 1995 and what had been proposed is that no EVA was necessary to do the 1995 mission. An adapter had been constructed to make it possible for the U.S. Space Shuttle to dock directly with the ”Mir” Space Station. The first Shuttle – Mir mission was followed by a number of docking missions involving both American astronauts and Russian cosmonauts. In this way, several Americans experienced truly long-duration spaceflights for the first time.
With the advent of the International Space Station, U.S. - Russian collaboration quickened. It is now quite routine to have “mixed” U.S. – Russian crews working for long periods of time on the International Space Station. Thus, in the span offour decades, sustaining life in space has evolved considerably from the first flights intended to prove that humans could endure microgravity. Since the first forays into space by Gagarin, Shepard, Grissom, Titov, and Glenn, the United States has explored the Moon, the Soviet Union/Russia has maintained a series of space stations in orbit for more than 25 years, and American astronauts have shepherded the orbital Shuttle through more than 100 missions and hundreds of sophisticated biomedical experiments. One of those missions carried Senator John Glenn, on his second flight 36 years after the Mercury 6 orbital flight, intended to underscore another NASA – NIH cooperation in the study of aging. An intrinsic, even critical, component of this evolution has been to define and overcome the biomedical challenges of human space flight and also to understand the role of gravity in critical life processes. After all, life originated and evolved under the constant pull of Earth’s gravity, using this force from cradle to grave in ways that are still poorly understood. Only recently, pulling together ground and flight data obtained from integrated and interdisciplinary biological and clinical experiments, scientists have begun to unravel the effects of gravity on living systems. This understanding was made possible by the collaboration of scientists around the world and the participation of the National Institutes of Health. The ability of gravity to up- or down-regulate certain genes involved in musculoskeletal metabolism and the capacity of the nervous system to adapt its function rapidly as a result of changing gravitational forces are just two of the most fascinating discoveries reported recently (4).
What follows is an attempt to present a concise overview of life in space, physiological responses to this new environment, and associated health implications for future space travelers. Consideration must be given to the hostile, and yet dynamic environment of space; to the craft that protects the crew members from the harsh environment, allowing them to navigate in space; and to life itself as it adjusts to this novel environment. Today we know that some adaptive changes, such as immune and hormonal responses, are primarily the response to the stresses of confinement, isolation, and spacecraft design, not necessarily to the unique effects of the space environment.
The Spaceflight Environment
The first astronauts and cosmonauts were required to function—eat, drink, communicate, and move—for extended periods of time in a novel and complex environment. Early biomedical studies demonstrated that the combined factors of spaceflight did exact a toll on the physical performance and health of crew members. Furthermore, early crew members and aerospace engineers realized that spaceflight was really the sum of several complex factors, only one of which is microgravity. The success of each mission is driven by a number of parameters—crew performance, crew health, the internal spacecraft environment, system performance, and the external environment—whose sum represents a challenge to safety and, most importantly, human survival.
External. In orbit, a spacecraft moves around Earth in a constant state of free fall that produces microgravity. Because all organisms on Earth have evolved and developed in the presence of gravity, the absence of this force imparts adaptive changes that are initiated immediately upon exposure. Some of these continue throughout the course of the mission. The net results of these changes are altered physiological function and structure that can offset health and performance in space and post flight.
Although the most conspicuous characteristic of the space flight environment is reduced gravity, a number of other factors contribute to its biomedical effects on humans. Primarily, space is a hostile environment to life. It is distinguished by profound fluctuations in temperature ranging from 220 K in the stratosphere to 1000 K in the thermosphere (5), the lack of a breathable atmosphere due to a near-near-perfect vacuum, and a number of constant and intermittent radiative events. In addition, the spacecraft is usually subjected to micrometeoroid bombardment and recently, to a large amount of human-made debris. All of these external and internal environmental events require constant monitoring to protect crew health and mission safety (6,7).
The radiative environment is comprised of several sources of ionizing radiation, a general term that encompasses particles that can alter molecular electrons upon contact: solar energetic particles emitted during solar flares, particles trapped in Earth’s magnetic field, and galactic cosmic radiation (8). Although each of these sources consists of various types of space radiative particles, the most significant barriers to mitigating radiative exposure are
1. an incomplete understanding of the detrimental effects caused by ionizing radiation, and
2. an inability to predict and model radiative events fully in time to protect a space-faring crew or biologically based life support system (9).
Presently, crew and spacecraft interior are monitored by a series of passive and active dosimeters, and ground-based monitoring can warn of impending solar flares so that the mission can be abbreviated. The Soviet Union and NASA performed a number of experiments, using biosatellites, the Space Shuttle, the Mir Space Station, and now the International Space Station (ISS), to understand the of effects protons, neutrons, electrons, and heavy cosmic ions on the function of living organisms. Now, it is postulated that one of the effects of continuous exposure to galactic – cosmic radiation can result in cellular genomic instability, manifested as generational cancer and/or malformations. Comparison of the measurements made during the Skylab mission in 1971-73 to those obtained from the NASA-MIR (ISS Phase I) experiments showed that the South Atlantic Anomaly, and the associated radiation, has moved westward and north, reflecting a displacement in Earth’s electromagnetic fields (4,9). NASA, together with the ISS partners, is now beginning to standardize radiative measurements and data analysis. Since 1998, NASA and the National Institutes of Health developed a research program to study the long-term genetic implications of exposures to constant low-level radiation. One of the most unexpected findings from the NASA – Russian cooperation was the realization that the spacecraft’s aluminum shell results in fragmentation and transformation of incoming energetic rays (bremsstrahlung) that contribute up to 30% of the total space radiative exposures (10).
Internal. Not all biomedical changes or medical events observed in spaceflight result from altered gravity or the influences of the external environment. The human-rated spacecraft must protect the crew from the hostile external environment and provide the resources necessary to support human life and work (see U.S. Manned Spaceflight: Mercury to the Shuttle). The design and performance of such spacecraft has evolved considerably from the first space capsules to the “shirt-sleeve” environment of the International Space Station. Common to all spacecraft environments, however, is the requirement for appropriate air, water, temperature, and pressure and consideration for the human factor in design and performances, that is, the environment must be precisely monitored and maintained using a minimum of resources including power, mass, and crew time (11).
Although the spacecraft itself must be rigorously designed to shield crew members from both constant and intermittent ionizing radiation, cumulative exposure levels within the spacecraft must be constantly monitored and assessed. Currently, both active and passive dosimeters of different sensitivities are used to track the exposure of each individual and selected regions in the spacecraft. As missions of increasing duration are emphasized, however, monitoring requirements will become more stringent, and highly accurate modeling and prediction will become a central means of protecting crew members. Because missions beyond earth’s orbit cannot be rescheduled or aborted to avoid predicted radiative events, exploratory missions will require a heavily shielded shelter, preferably constructed with a nonmetallic and high hydrogen content material within the spacecraft, or some other solution for protecting space-faring crews from high radiative exposures.
The spacecraft environment itself provides additional challenges to crew health and safety. A majority of these constraints results from the combined physical environment of spaceflight. Crew members are expected to live in confinement far distant from friends and family are subjected to extreme scrutiny and pressure to complete their work in a timely and consistent manner (12). This stress and isolation is compounded by the fact that a sunrise or sunset occurs every 90 minutes in low Earth orbit. Although initially disconcerting to crew members, altered dark-light cycles have a significant physiological effect on the quality and quantity of sleep and ultimately, performance. The sum of these challenges, both mental and physical, can exact a considerable toll on crew members unless they have appropriate support from ground personnel, sufficient personal time and space, and flexible work/rest schedules.
Numerous analog environments, including extended bed rest and Antarctic wintering-over expeditions, have been explored to understand the cumulative effects on human performance and psychosocial health. Human experimentation in space is a tedious and difficult task: differences among missions, variability in environmental parameters, small sample size, and mission constraints have made the design and execution of controlled experiments quite challenging (6). The Soviet Union/Russia operated its spacecraft at near 760 torr and normal atmospheric gas composition and pressure, whereas the United States adopted a one-gas (100% oxygen) one-third atmospheric (250 torr) pressure. The intent was to simplify the life support system and minimize decompression sickness during space walks. This approach was used for early human missions and was intended to save time in the race to the Moon. At the conclusion of the Apollo missions, NASA engineers were faced with a prospect of oxygen toxicity during the long-duration Skylab missions. NASA and U.S. Air Force life scientists solved this problem by adding 20% nitrogen to Skylab’s atmosphere. In the meantime, the tragic death of the three Soviet cosmonauts returning from the first Space Station, Salyut 1, created concerns in NASA regarding the safety of long-duration biomedical research on Skylab. Following a cooperative agreement signed in 1971, Soviet life scientists shared the medical findings from the Salyut 1 mission with their NASA counterparts, demonstrating that rapid decompression, due to a hatch seal failure, resulted in crew death. This mutual collaboration ensured confidence and the success of Skylab missions, and it also generated an unprecedented and very successful scientific and professional medical relationship between the physicians and scientists of the two countries. This relationship persists to this day, despite many political and management changes in both countries. The major engineering and biomedical challenge to both countries was presented during the planning and execution of the Apollo-Soyuz Test Project, docking two spacecraft that had markedly dissimilar atmospheres, posing a potential fire hazard to the Soyuz Spacecraft, and the risk of bends to the crews. A special transfer airlock module was developed for this purpose. This module was carried by the Apollo spacecraft and docked with the Soyuz; during the joint phase, the atmospheric pressure in the Soyuz was reduced by 155 torr, and the oxygen concentration was slightly increased. During a 3-day period, the crews visited each other’s spacecraft three times; no bends occurred despite repeated recompressions and decompressions in the airlock.
The Space Shuttle presented a real challenge for space walks and the risk of bends. NASA adopted a standard atmosphere composition and pressure, in a decision made in response to the demands from the international scientific community, but the space suit pressure and gas composition changed little. NASA’s budgetary constraints precluded the opportunity of using advanced space suits designed by the Ames Research Center. This concern for bends continues to persist in the ISS era.
Over the years, life scientists learned that an answer to the challenges posed by the engineering design, mission constraints, and uncertainties of research in space is to use multiple species, in addition to humans, to document similarities in responses to experimental variables among species. This requires integrating many different biological specimens into major life science experiments, especially during the Shuttle/Spacelab era. The necessity to execute integrated international experiments to increase the scientific return was demonstrated by the U.S. and Soviet/Russian life science cooperation (4,10).
To date, more than 350 individual biological experiments were flown in space. NASA launched 20 suborbital and 4 orbital missions, and Soviet Union/ Russia launched more than 22 spacecraft (Biosattelite) dedicated primarily to fundamental biology. The last 11 Biosatellites included international participation, with major contributions from the NASA Ames Research Center. A total of 35 different species were flown by NASA, Russia, Europe, Japan, Ukraine, and Canada. The results of these investigations are presented in the following sections.
Biomedical Challenges
The organism uses a complex set of biological tools to sense, process, and respond to the ever-changing environment, be it the normal habitat on Earth or the close quarters of spacecraft. Full understanding of the relationships between these various tools is yet to be achieved. Table 1 presents concisely the sum of our current knowledge in this area.
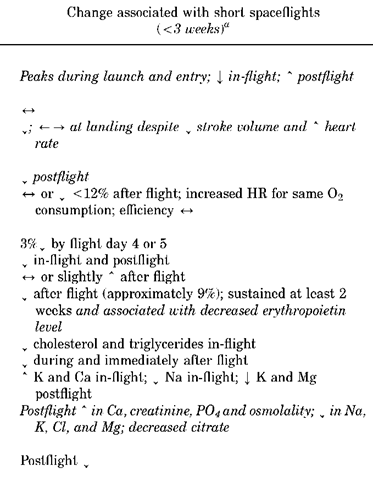
Because of the complexity and individual nature of human adaptation to spaceflight, changes are often considered on a systemic basis. Although this approach is useful for understanding system-specific functional alterations, it does not fully characterize the effects of living and working in the spaceflight environment. As a result, more recent studies have focused on whether crew members maintain appropriate levels of functional performance, the ability to perform key activities such as intra- and extravehicular activity (IVA, EVA) or emergency egress during or following long-duration spaceflight. Aerobic capacity, a measure of the amount of oxygen consumed during a single bout of maximal exercise, reflects the integrated performance of the cardiovascular, nervous, and musculoskeletal systems. As illustrated in Fig. 1, aerobic capacity is diminished by spaceflight but does show a relatively rapid return toward preflight levels upon return to Earth (6,13).
Cardiovascular Deconditioning. The cardiovascular system has evolved in the presence of gravity as an intricate network of vasculature that contains blood and is powered by the heart. This vasculature is composed of both muscular arterial vessels that supply oxygenated blood to tissue and nonmuscular venous vessels that return blood to the heart. Baroreceptors and stretch-sensitive receptors monitor the critical parameter of blood pressure in vessels throughout the body and adapt to postural changes. On Earth, simple motions such as sitting, standing, or reclining result in significant and rapid responses to changes in gravitational force imposed on the body.
Spaceflight presents a challenge to the cardiovascular system that is generally stabilized by the fifth week of flight. In even longer Soviet space flights (3 months to 1 year or more), a slight increase in heart rate has been noted, particularly toward the end of the mission (7,14). Nevertheless, cardiovascular deconditioning, by Earth’s standards, appears to be a self-limiting phenomen on that does not worsen with flight duration (unless other medical conditions, such as dehydration or infection prevail) and does improve upon return to Earth. In space, cardiovascular responses represent an appropriate adjustment to a new environment, in which the gravitational load placed on the heart is considerably less than on Earth. Fluid pooling no longer occurs in the lower extremities but is instead localized to the upper body. Physically, this shift is revealed by facial edema, sinus congestion, decreased calf girth, and leg volume (”bird legs”). This shift is perceived as excess fluid, which in turn affects a series of immediate but long-lasting changes. An immediate decrease in plasma volume occurs, in addition to a more gradual loss of approximately 10% of red blood cell mass (15). This condition is primarily attributed to a decrease in circulating blood volume. Systematic investigations have proven difficult because of individual differences in diet, sleep patterns, exercise, medications, and fluid intake associated with various space missions. Recent studies have focused on secretion ofhormones (such as norepinephrine), baroreceptor changes with time, and the role of the central nervous system in regulating of the cardiovascular system (16).
Table 1. A Summary of the Physiological Changes Associated with Human Spaceflight
Figure 1. Aerobic capacity of crew members before launch (L — 10 days) and after flight (Return, Day 0 and Return, Day 3) as measured by maximum O2 uptake in liters per minute during an exercise stress test. Control subjects did not perform any exercise, whereas CM subjects exercised using an onboard cycle ergometer up to 48 hours before landing (13).
Microgravity-induced cardiovascular adaptation becomes a medical problem only after crew members are subjected to accelerative forces during reentry or upon return to the constant 1-g stress on Earth. As early as the American Gemini program, cardiovascular deconditioning was documented in 100% of crew members. One component of this deconditioning is orthostatic intolerance, the inability to function effectively against gravitational stress, such that simple actions like sitting and standing may result in episodes of weakness, dizziness, or fainting. A standard measure of orthostatic intolerance is the stand test, in which recently returned crew members are asked to stand upright for several minutes after a period of reclining; by monitoring blood pressure and heart rates during this functional challenge, researchers can associate significantly altered arterial pressures with adaptation to space flight and the gravitational forces of landing. As shown in Fig. 2, approximately 20% of crew members showed altered levels of systolic and diastolic pressure following flight. Depending on the duration of the space flight and the amount of exercise performed in flight, the return of cardiovascular function to preflight values may take as long as 2 weeks. Routinely used countermeasures include aerobic exercise, fluid and electrolyte replenishment (especially before return to Earth), and exposure to simulated gravity via the lower body negative pressure device (6,7,17,18). Neurosensory Disturbances. The central nervous system (CNS) controls both perception of and interaction with the environment. The sensory system, including the visual, vestibular, and proprioceptive organs, responds to environmental stimuli and supplies a constant flow of input to the CNS. In conjunction with a visual image of surroundings, the vestibular and proprioceptive systems supply additional information relating to orientation, balance, and limb location. The CNS processes this information and then directs the musculoskeletal system’s movement and interaction with the environment. Each step in this intricate process is contingent upon a constant inflow of information about the surrounding environment. In microgravity, however, the CNS must adapt to a loss of sensory and proprioceptive input, and it also must also respond to reduced muscular capacity, including functional and structural changes in muscle tissue. Adaptation to unexpected or even absent sensory information is neither an instantaneous nor a constant process; thus identifying the mechanisms responsible and the appropriate countermeasures is somewhat of a challenge. Neurosensory adaptations have traditionally been difficult to measure but indirectly are evidenced through in-flight and postflight changes in crew performance.
Figure 2. Systolic and diastolic pressure responses of crew members to entry, landing, and egress shows altered reactions to the standard orthostatic challenge, the stand test. Blood pressure was measured preflight while seated and standing. The middle section of this graph represents blood pressure values in-orbit (an average of 130/76 mm Hg), during reentry, and until touchdown and egress from the orbiter seat. Values while seated in the orbiter are much higher than preflight values (16).
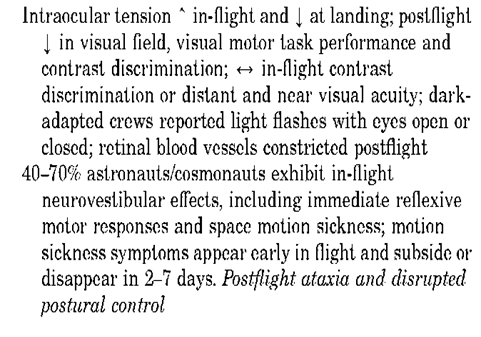
Clinically, the most important vestibular disturbance associated with spaceflight is space motion sickness (SMS). As Titov noted, most crew members do experience a sensation of bodily inversion, which soon passes but can recur due to rapid movement (19). More susceptible individuals, however, develop a full host of SMS symptoms (20). Russian and U.S. demographics suggest that SMS affects 40-70% of astronauts and cosmonauts, depending on the classification of symptoms. SMS occurs early in the mission, typically within the first 3 days. Symptoms range from minimal discomfort to nausea and vomiting, accompanied in rare cases by pallor and sweating. Head and body movements tend to worsen the discomfort. When the symptoms are severe, crew performance can be affected and mission efficiency severely compromised. During the Apollo IX mission, for example, certain crew activities were delayed by 24 hours due to space motion sickness.
The medical basis for space motion sickness is not fully understood, partly because the phenomenon can be studied effectively only during spaceflight. Guedry et al. (21) summarized studies of motion sickness in flight and on the ground, in which the most plausible explanation for neurosensory changes is the ”sensory conflict” hypothesis. According to this concept, the usual sensory inputs to the vestibular receptors of the inner ear are no longer present in microgravity, causing altered processing of sensory information and ultimately resulting in altered motor responses. A series of elegant biological experiments conducted aboard the dedicated Spacelab Life Sciences Missions 1 and 2 and the NASA-NIH Neurolab Mission, dedicated to the Decade of the Brain, demonstrated the plasticity of the CNS in response to altered gravitational forces. The CNS does develop new coping strategies in novel environments, which facilitates orientation and navigation. Of interest is the fact that individuals who use internal cues for orientation fare better in the SMS manifestation and adaptation process than those use external cues. Repeated exposures to the space environment reduce the severity of SMS and the length of time required to adapt. Anecdotal data suggests that women are less susceptible to SMS. Finally, Earth-based simulators cannot identify individuals susceptible to SMS. Absorption of orally ingested medications is altered in space, and drugs are not effective in preventing or treating SMS. Injectable PhenerganTM has been successfully used to treat SMS in-flight and has minimal side effects (6).
Both the Russian and American human spaceflight programs recognize the complex interactions within and between the sensory-motor, nervous, cardiovascular, and muscular systems (10). A significant finding from postflight research is the ataxia present during ambulation in the majority of flown individuals. Current countermeasures against this ataxia involve resistive exercise combined with compressions along the longitudinal axis of the body. Musculoskeletal Alterations. An integrated response from the skeletal, muscular, connective tissue, and nervous systems permits movement in a 1-g environment. This response is predicated on the fact that certain directional forces must be overcome to complete ordinary tasks, such as lifting an object or walking down stairs. In the microgravity environment, however, these directional forces are altered; the result is a cascade of functional and structural changes in the physiological systems that control locomotor tasks. Collectively, these changes yield reductions in strength, power, and endurance that ultimately influence crew-members’ ability to perform routine motor activities (Table 2). The changes are ordinarily indicated in flight by a progressive decrease in total body mass, leg volume, and muscular strength. As weight-bearing muscles and bones adapt to the microgravity environment, several symptoms are manifested. Disturbances in postural and motor coordination, locomotion function, and equilibrium can be seen, and alterations in proprioceptor activity and spinal reflex mechanisms occur. Although all of these changes appear to be dependent, at least to some extent, on flight duration, they have been reversible, and no adverse sequelae have been reported thus far.
Table 2. Mean Strength Performance of Skeletal Muscle on Landing versus Preflight (n = 17) during Concentric and Eccentric (Extension) Motions of Selected Muscle Groups (13) Pre> Landing (p<0.05)
| Muscle group | Test mode | ||
| Concentric | Eccentric | ||
| Back
Abdomen Quadriceps Hamstrings Tibialis Anterior Gastroc/Soleus Deltoids Pecs/Lats Biceps Triceps |
 |
 |
A primary indicator of changes in bone and muscle is body mass: in-flight weight losses of 3-4% were seen in early, short-duration spaceflights. With the advent of longer missions, most weight loss took place during the first three to five flight days, and a much more gradual decline thereafter (22). The findings suggest that a significant part of the initial change in body mass is due to the loss of fluids, either through diuresis or decreased thirst and fluid intake (18), and that subsequent losses are due to metabolic imbalances and/or muscle atrophy. These changes appear to be self-limiting, the largest weight losses recorded (6 to 7 kg) are independent of mission duration. In more recent long-duration space missions, where adequate caloric intake and physical exercise have been maintained by some crew members, actual weight gains have been reported. Such weight gains probably reflect an overall increase in fatty tissue, which was more than sufficient to offset losses of muscle tissue (7,10). In any event, body mass lost in flight is rapidly regained in the postflight period.
Muscle Atrophy. Muscle atrophy results from structural and functional changes in muscles. These changes are most readily apparent in the postural or antigravity muscles, such as the gastrocnemius abdominal, back, and neck muscles. Skeletal muscles exhibit numerous alterations in strength and endurance properties, including force- and power-generating capacities, shortening and relaxing rates, neural activation patterns, protein expression, and metabolic utilization profiles. Concomitant with these muscular changes, connective tissues undergo similar atrophy and functional alteration. At the molecular level, both slow-twitch and fast-twitch muscle fibers are affected. The process of functional and structural change is progressive and can be controlled to some extent by increasing caloric intake, dietary adjustments, and intensive strength exercises.
Evidence of the deterioration of muscle during spaceflight comes from several sources. In-flight measurements of leg volume (Fig. 3) show an initial rapid decrease that can be attributed to the headward fluid shift and is followed by gradual recovery. Post flight biostereometric measurements of Skylab astronauts demonstrated more general losses of volume from the abdomen downward, although losses in the abdomen and buttocks were attributed to the loss of fat (23). Postflight urinary analyses reveal in-flight increases in the excretion of a number of metabolites associated with muscle breakdown, such as nitrogen, potassium, creatine, and amino acids. Metabolic balance studies and electromyo-graphic analyses of muscular activity further substantiate the deterioration of muscle function during spaceflight. Electromicroscopic analysis of human and rodent muscle biopsies showed decreased production of slow-twitch myosin fibers (endurance) and normal distribution of fast-twich myosin fibers (dexterity). Additional investigations performed on rodents flown on Spacelab missions demonstrated the selective effects of gravity on the type of muscle myosine production (4).
Figure 3. Percent decreases in volumes of various leg muscles, measured by magnetic resonance imaging, from three extended duration Orbiter Shuttle missions (13).
Bone and Mineral Changes. Removing muscular forces and weight from bones, as occurs in bed rest or having a limb in a cast, causes a loss of bone mineralization, known as disuse osteoporosis. During space flight, crew members experience a form of musculoskeletal disuse in which levels of bone mineral are decreased. Early studies of bone mineral changes using X-ray densitometry suggest that large amounts of bone may be lost during relatively brief periods of spaceflight, and countermeasures to this loss are mandatory for long-duration missions (24). The 12 crew members who participated in the Gemini 4, 5, and 7 and Apollo 7 and 8 missions averaged 3.2% postflight losses of bone density from the calcaneus (heel bone) compared with preflight baseline values. Some losses were also observed from the radius and ulna after these early flights. Data from Soviet/Russian cosmonauts and U.S. astronauts who flew on the Mir station show a continued loss of 1% of bone mass per month, even using exercise as a coun-termeasure. Resistive exercise, hormone therapy, and drugs such as bisphosphonates are currently being evaluated as potential countermeasures to bone loss.
In sum, these changes to the locomotion system means that crew members are at risk for increased falls, bone fractures, and limited mobility—conditions, which at a minimum, could make emergency egress a challenge. Immunologic Alterations. The immune system defends the body against any cell, substance, or organism not recognized as self. As such, it is affected by both environmental and physiological fluctuations that occur during spaceflight. Although results from some studies are contradictory, most generally recognize an increase in the immune cells responsible for the immune response, known as leukocytes. More specifically, changes in the leukocyte population, particularly in the relative percentage of T and B lymphocytes, are altered compared to preflight levels. Lymphocytes from astronauts on board Soyuz 6, 7, and 8, Skylab 2, 3, and 4, and Salyut 4 exhibited poor response to mitogenic factors (25), the substances that induce the immune response. Therefore, the cells, experience a reduced functional capacity in microgravity conditions.
The immune system, like other body systems, responds dynamically to varying conditions, which may explain why results from one study contradict those of others. Studies conducted as early as the Skylab program suggest that impaired immune function during spaceflight is closely linked to the endocrine system and is particularly affected by corticosteroids and catecholamines (25); generally, this implies that changes in other regulatory mechanisms could closely affect immune function. In addition, results from in vitro studies may not parallel results from in vivo studies, indicating that the physiological environment plays an integral role in maintaining immunologic integrity (26).
It has been shown in several studies that stress has a considerable influence on immunity. Astronauts experience psychological and physical stresses that may result in reactivating latent viruses during space flight, potentially increasing the risk of infection among the crew. A study done on the amount of Epstein-Barr shedding pre-, in- and postflight on the Shuttle and Mir crews, showed that the virus, normally latent in most humans, was higher in samples taken before launch (27). Although results from this study suggest that stress levels are higher before than during or after flight, reactivation of latent viruses, combined with depressed immune response in flight, poses a threat to both short-and long-term missions in the event of injury or infections.
Because immune function and activity are interdependent on other systems of the body, there are implications for exploring countermeasures to mitigate the changes and for studying how pharmacological substances interact with the immune system in microgravity (18). Similar changes in immune response were reported in wintering personnel in Antarctica and, it is thought, are due to confinement and isolation. Carefully planned studies will be required to shed further light on this important issue, which could affect the development of chronic and debilitating diseases.
Hematologic, Fluid, and Electrolyte Changes. The headward shift of fluids in weightlessness and the resulting decrease in circulating blood volume are responsible for many of the physiological changes that occur during adaptation to spaceflight conditions. As has been discussed, they directly affect the functioning of the cardiovascular system. They also have several effects on the composition of body fluids, especially blood (Table 1). The most significant hematologic changes involve a reduction in plasma volume, alterations in red blood cell (RBC) mass,and changes in the distribution of RBC shapes. From the time of the early Gemini and Vostok missions, a postflight decrease in total RBC mass has been observed in nearly all U.S. and Soviet crew members. There is a gradual decrease, losses average about 9% of the total RBC pool during the first 30 to 60 days in flight, and values range from 2-21%. Cosmonauts who participated in missions of 18 days to 6 months have shown a postflight decrease in erythrocyte counts that returned to baseline values within 6 weeks (22).
The magnitude of the RBC loss does not appear to relate to the length of missions longer than 14 days. Changes in RBC are also accompanied by changes in the shapes of erythrocytes, although these alterations do not seem to affect crew health or function in flight and are rapidly reversed postflight. The weight of evidence now suggests that the loss of RBC mass is due, instead, to insufficient circulating erythropoietin in combination with neocytolysis, or a decreased survival rate of newly formed RBCs (7,15). The decrease in RBC mass is effectively masked by a simultaneous, rapid decline in plasma volume (4-16% from preflight values) such that the ratio of cells to plasma remains roughly normal (18).
The microgravity-induced fluid shift produces at least a transient increase in central blood volume (18). Research from ground-based bed-rest studies suggests that the stretch receptors in the left atrium interpret this as an increase in total circulating blood volume and trigger a compensatory loss of water, sodium, and potassium from renal tubules. This is the first event in a series of fluid and electrolyte shifts that occur during the adaptation to weightlessness. So far, early diuresis has been observed only in bed-rest studies. It is difficult to demonstrate during spaceflight because of the problems involved in accurately documenting urine volumes early in flight while water intake is usually reduced due to SMS. Additional findings include in-flight increases in the urinary output of sodium, potassium, and chloride, an in-flight decrease in antidiuretic hormone, and reduced postflight excretion of sodium. Fluid retention has also been a consistent finding in cosmonauts after Soyuz flights, but it was found that excretion of potassium and calcium increases. Alterations in electrolytes are believed responsible for cardiac arrhythmias on Apollo 15 and subsequent U.S. and Soviet missions (7).
Psychological Health. The spaceflight environment consists of many elements that, even if experienced separately, are both physically and mentally challenging. A confined living space, high public interest and visibility, isolation from family and friends, crowded or often unappealing spacecraft conditions, and requirement for strong group dynamics are but a few of the obstacles that cosmonauts and astronauts face. These factors are compounded by the physical fact that light/dark cycles are altered in orbit, which affects circadian rhythm and is evidenced by disrupted or insufficient sleep (28). The most prevalent psychological events reported by crew members include high levels of stress or tension, anxiety often demonstrated as annoyance at other crew members or ground support personnel, decreased levels of concentration, emotional instability including mood elevation or depression, and general fatigue.
As increasingly longer missions become feasible, psychological and behavioral support has become an element of both the American and Russian space medicine programs. Often, support takes the form of comparatively small changes in operations or scheduling that minimize crew requirements and permit crew members some flexibility in arranging their work/rest cycles. For example, astronauts have reported considerable improvements in outlook and performance when communications with family members or friends are provided regularly. Careful planning for work/rest cycles, proper recreation, nutrition, and interpersonal and family communications are essential in maintaining psychological health during long-duration missions. The Soviet/Russian program has developed an elaborate system for its crews, which is now adapted to the ISS program (7).
Time Course of Adaptations
The human body is exquisitely sensitive to changes in its surroundings and reacts to such changes with equal precision. Modest changes in gravitational force, for example, as a sitting person stands or a sleeping person awakens induce a host of regulatory or adaptive mechanisms to ensure that blood consistently reaches all extremities. A more significant change to the gravitational environment—such as the microgravity of spaceflight—clearly challenges the body’s homeostasis to a much greater extent (6).
The earliest orbital flights were conducted in small capsules and lasted only a few hours or days. Within these first human-rated spacecrafts, the limited capacity for movement and the short exposure to microgravity meant that crew members mainly reported rapid onset of adaptation (1). As mission duration increased well beyond several days into months and even years, crews are now faced with further adaptive events and new physiological challenges; adaptation to spaceflight is neither instantaneous nor consistent, but instead depends on individuals, mission duration, and operational activities (6). Despite these differences, all crew members who return from both short- and long-duration flights report two periods of adaptation that occur after the transition from one gravitational environment to another.
The first is experienced upon launch and entry into orbit. Some symptoms manifested early in the mission abate as adaptation is resolved. The sensory conflict produced by the visual and vestibular systems is one example that is limited to the first 3 to 8 days of a mission.
The return to Earth’s gravity requires a second period of adaptation, which again presents a significant challenge to crew activity and safety. Orthostatic intolerance stems from the cardiovascular deconditioning and cephalic fluid shift that occurs in response to microgravity; many crew members report presyncopal or syncopal episodes, that is, dizziness or fainting, upon return to 1g. Neuromuscular and neurovestibular adaptations produce post flight disequilibrium (including marked vertigo in some cases) and gait disturbances; both clearly limit coordinated maneuvers and interfere with nominal or contingency egress (13,21). Cosmonauts from long-duration Russian missions of 8 months have required more than 4 weeks of rehabilitation to function normally (29). Physical performance also declines as a result of significant and sustained loss of bone and muscle mass, documented at 10-20% of preflight levels during extended-duration missions.
Bone and connective tissue changes, for example, begin as early as 1 week into a mission and can continue for more than a year. These changes are not typically apparent in flight, but are instead demonstrated upon return to Earth as locomotor problems, bone frailty, and increased risk of kidney stones (22).
Challenges for Exploration-Class Missions
As human spaceflight continues beyond low Earth orbit, health monitoring and health maintenance through appropriate countermeasures will become more discrete and seamless in the spacecraft of the future. Crewmembers may well monitor their own medical status, evaluate environmental health, assess risks, and then direct automatic correction or restoration of an anomaly. The opportunity for novel or previously unexplored countermeasure approaches, including artificial gravity, could well alter what are currently considered the most dire biomedical challenges of human spaceflight. The crew of the International Space Station and future spacefarers will be just as dependent as their forebears on a thorough understanding and mitigation of these challenges (30).