Definition
Vasculitis is a clinicopathologic process characterized by inflammation of and damage to blood vessels. The vessel lumen is usually compromised, and this is associated with ischemia of the tissues supplied by the involved vessel. A broad and heterogeneous group of syndromes may result from this process, since any type, size, and location of blood vessel may be involved. Vasculitis and its consequences may be the primary or sole manifestation of a disease; alternatively, vasculitis may be a secondary component of another primary disease.Vasculitis may be confined to a single organ, such as the skin, or it may simultaneously involve several organ systems.
Classification
A major feature of the vasculitic syndromes as a group is the fact that there is a great deal of heterogeneity at the same time as there is considerable overlap among them. This heterogeneity and overlap in addition to a lack of understanding of the pathogenesis of these syndromes have been major impediments to the development of a coherent classification system for these diseases. Table 10-1 lists the major vasculitis syndromes.The distinguishing and overlapping features of these syndromes are discussed below.
Pathosphysiology and Pathogenesis
Generally, most of the vasculitic syndromes are assumed to be mediated at least in part by immunopathogenic mechanisms that occur in response to certain antigenic stimuli (Table 10-2). However, evidence supporting this hypothesis is for the most part indirect and may reflect epiphenomena as opposed to true causality. Furthermore, it is unknown why some individuals might develop vasculitis in response to certain antigenic stimuli, whereas others do not. It is likely that a number of factors are involved in the ultimate expression of a vasculitic syndrome. These include the genetic predisposition, environmental exposures, and the regulatory mechanisms associated with immune response to certain antigens.
Pathogenic Immune-Complex Formation
Vasculitis is generally considered within the broader category of immune-complex diseases that include serum sickness and certain of the connective tissue diseases, of which systemic lupus erythematosus is the prototype.
TABLE 10-1
VASCULITIS SYNDROMES
|
PRIMARY VASCULITIS |
SECONDARY VASCULITIS |
|
SYNDROMES |
SYNDROMES |
|
Wegener’s granulomatosis |
Drug-induced vasculitis |
|
Churg-Strauss syndrome |
Serum sickness |
|
Polyarteritis nodosa |
Vasculitis associated with other primary diseases |
|
Microscopic polyangiitis |
|
|
Giant cell arteritis |
Infection |
|
Takayasu’s arteritis |
Malignancy |
|
Henoch-Schönlein purpura |
Rheumatic disease |
|
Idiopathic cutaneous vasculitis |
|
|
Essential mixed cryoglobulinemia |
|
|
Behget’s syndrome |
|
|
Isolated vasculitis of the central nervous system |
|
|
Cogan’s syndrome |
|
|
Kawasaki disease |
Although deposition of immune complexes in vessel walls is the most widely accepted pathogenic mechanism of vasculitis, the causal role of immune complexes has not been clearly established in most of the vasculitic syndromes. Circulating immune complexes need not result in deposition of the complexes in blood vessels with ensuing vasculitis, and many patients with active vasculitis do not have demonstrable circulating or deposited immune complexes. The actual antigen contained in the immune complex has only rarely been identified in vas-culitic syndromes. In this regard, hepatitis B antigen has been identified in both the circulating and deposited immune complexes in a subset of patients with systemic vasculitis, most notably in polyarteritis nodosa (PAN; see below).The syndrome of essential mixed cryoglobulinemia is strongly associated with hepatitis C virus infection; hepatitis C virions and hepatitis C virus antigen-antibody complexes have been identified in the cryoprecipitates of these patients (see below).
TABLE 10-2
POTENTIAL MECHANISMS OF VESSEL DAMAGE IN VASCULITIS SYNDROMES
|
Pathogenic immune complex formation and/or deposition Henoch-Schönlein purpura |
|
Vasculitis associated with collagen vascular diseases |
|
Serum sickness and cutaneous vasculitis syndromes |
|
Hepatitis C-associated essential mixed cryoglobulinemia |
|
Hepatitis B-associated polyarteritis nodosa |
|
Production of antineutrophilic cytoplasmic antibodies |
|
Wegener’s granulomatosis |
|
Churg-Strauss syndrome |
|
Microscopic polyangiitis |
|
Pathogenic T lymphocyte responses and granuloma formation |
|
Giant cell arteritis |
|
Takayasu’s arteritis |
|
Wegener’s granulomatosis |
|
Churg-Strauss syndrome |
The mechanisms of tissue damage in immune complex-mediated vasculitis resemble those described for serum sickness. In this model, antigen-antibody complexes are formed in antigen excess and are deposited in vessel walls whose permeability has been increased by vasoactive amines such as histamine, bradykinin, and leukotrienes released from platelets or from mast cells as a result of IgE-triggered mechanisms. The deposition of complexes results in activation of complement components, particularly C5a, which is strongly chemotactic for neutrophils. These cells then infiltrate the vessel wall, phagocytose the immune complexes, and release their intracytoplasmic enzymes, which damage the vessel wall. As the process becomes subacute or chronic, mononuclear cells infiltrate the vessel wall. The common denominator of the resulting syndrome is compromise of the vessel lumen with ischemic changes in the tissues supplied by the involved vessel. Several variables may explain why only certain types of immune complexes cause vasculitis and why only certain vessels are affected in individual patients. These include the ability of the reticuloendothelial system to clear circulating complexes from the blood, the size and physicochemical properties of immune complexes, the relative degree of turbulence of blood flow, the intravas-cular hydrostatic pressure in different vessels, and the preexisting integrity of the vessel endothelium.
Antineutrophil Cytoplasmic Antibodies (ANCA)
ANCA are antibodies directed against certain proteins in the cytoplasmic granules of neutrophils and monocytes. These autoantibodies are present in a high percentage of patients with certain systemic vasculitis syndromes, particularly Wegener’s granulomatosis and microscopic polyangiitis, and in patients with necrotizing and crescentic glomerulonephritis. There are two major categories of ANCA based on different targets for the antibodies. The terminology of cytoplasmic ANCA (cANCA) refers to the diffuse, granular cytoplasmic staining pattern observed by immunofluorescence microscopy when serum antibodies bind to indicator neutrophils. Proteinase-3, the 29-kDa neutral serine proteinase present in neutrophil azurophilic granules, is the major cANCA antigen. More than 90% of patients with typical active Wegener’s granulomatosis have detectable antibodies to proteinase-3 (see below). The terminology of perinuclear ANCA (pANCA) refers to the more localized perinuclear or nuclear staining pattern of the indicator neutrophils. The major target for pANCA is the enzyme myeloperoxidase; other targets that can produce a pANCA pattern of staining include elastase, cathepsin G, lactoferrin, lysozyme, and bactericidal/ permeability-increasing protein. However, only antibodies to myeloperoxidase have been convincingly associated with vasculitis. Antimyeloperoxidase antibodies have been reported to occur in variable percentages of patients with microscopic polyangiitis, Churg-Strauss syndrome, crescentic glomerulonephritis, anti-glomerular basement membrane disease (Goodpasture’s syndrome), and Wegener’s granulomatosis (see below).A pANCA pattern of staining that is not due to antimyeloperoxidase antibodies has been associated with nonvasculitic entities such as rheumatic and nonrheumatic autoimmune diseases, inflammatory bowel disease, certain drugs, and infections such as endocarditis and bacterial airway infections in patients with cystic fibrosis.
It is unclear why patients with these vasculitis syndromes develop antibodies to myeloperoxidase or proteinase-3, whereas such antibodies are rare in other inflammatory diseases and autoimmune diseases. There are a number of in vitro observations that suggest possible mechanisms whereby these antibodies can contribute to the pathogenesis of the vasculitis syndromes. Proteinase-3 and myeloperoxidase reside in the azurophilic granules and lysosomes of resting neutrophils and monocytes, where they are apparently inaccessible to serum antibodies. However, when neutrophils or monocytes are primed by tumor necrosis factor (TNF) α or interleukin (IL) 1, proteinase-3 and myeloperoxidase translocate to the cell membrane where they can interact with extracellular ANCA. The neutrophils then degranulate and produce reactive oxygen species that can cause tissue damage. Furthermore, ANCA-activated neutrophils can adhere to and kill endothelial cells in vitro. Activation of neutrophils and monocytes by ANCA also induces the release of proinflammatory cytokines such as IL-1 and IL-8. Recent adoptive transfer experiments in genetically engineered mice provide further evidence for a direct pathogenic role of ANCA in vivo. In contradiction, however, a number of clinical and laboratory observations argue against a primary pathogenic role for ANCA. Patients may have active Wegener’s granulomatosis in the absence of ANCA; the absolute height of the antibody titers does not correlate well with disease activity; and patients with Wegener’s granulomatosis in remission may continue to have high antiproteinase-3 (cANCA) titers for years (see below).Thus, the role of these autoantibodies in the pathogenesis of systemic vasculitis remains unclear.
Pathogenic T Lymphocyte Responses and Granuloma Formation
In addition to the classic immune complex-mediated mechanisms of vasculitis as well as ANCA, other immuno-pathogenic mechanisms may be involved in damage to vessels. The most prominent of these are delayed hypersensitivity and cell-mediated immune injury as reflected in the histopathologic feature of granulomatous vasculitis. However, immune complexes themselves may induce granulomatous responses. Vascular endothelial cells can express HLA class II molecules following activation by cytokines such as interferon (IFN) γ. This allows these cells to participate in immunologic reactions such as interaction with CD4+ T lymphocytes in a manner similar to antigen-presenting macrophages. Endothelial cells can secrete IL-1, which may activate T lymphocytes and initiate or propagate in situ immunologic processes within the blood vessel. In addition, IL-1 and TNF-α are potent inducers of endothelial-leukocyte adhesion molecule 1 (ELAM-1) and vascular cell adhesion molecule 1 (VCAM-1), which may enhance the adhesion of leukocytes to endothelial cells in the blood vessel wall. Other mechanisms such as direct cellular cytotoxicity, antibody directed against vessel components, or antibody-dependent cellular cytotoxicity have been suggested in certain types of vessel damage. However, there is no convincing evidence to support their causal contribution to the pathogenesis of any of the recognized vasculitic syndromes.
Approach to the Patient:
Vasculitis
The diagnosis of vasculitis is often considered in any patient with an unexplained systemic illness. However, there are certain clinical abnormalities that when present alone or in combination should suggest a diagnosis ofvasculitis.These include palpable purpura,pulmonary infiltrates and microscopic hematuria, chronic inflammatory sinusitis, mononeuritis multiplex, unexplained ischemic events, and glomerulonephritis with evidence of multisystem disease. A number of nonvasculitic diseases may also produce some or all of these abnormalities. Thus, the first step in the workup of a patient with suspected vasculitis is to exclude other diseases that produce clinical manifestations that can mimic vasculitis (Table 10-3). It is particularly important to exclude infectious diseases with features that overlap those of vasculitis, especially if the patient’s clinical condition is deteriorating rapidly and empirical immunosuppressive treatment is being contemplated.
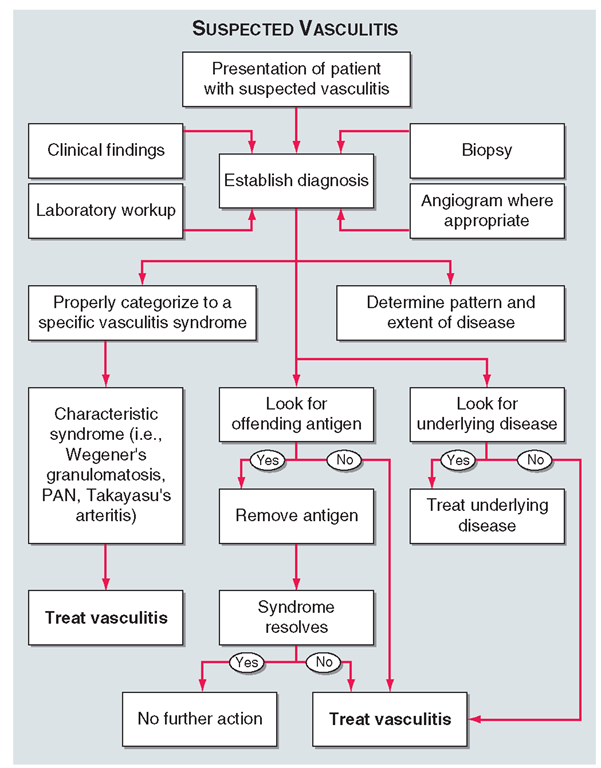
Once diseases that mimic vasculitis have been excluded, the workup should follow a series of progressive steps that establish the diagnosis of vasculitis and determine, where possible, the category of the vasculitis syndrome (Fig. 10-1).This approach is of considerable importance since several of the vasculitis syndromes require aggressive therapy with glucocorticoids and cytotoxic agents, while other syndromes usually resolve spontaneously and require symptomatic treatment only. The definitive diagnosis of vasculitis is made upon biopsy of involved tissue. The yield of “blind” biopsies of organs with no subjective or objective evidence of involvement is very low and should be avoided. When syndromes such as PAN, Takayasu’s arteritis, or isolated central nervous system (CNS) vasculitis are suspected, angiogram of organs with suspected involvement should be performed. However, angiograms should not be performed routinely when patients present with localized cutaneous vasculitis with no clinical indication ofvisceral involvement.
TABLE 10-3
|
CONDITIONS THAT CAN MIMIC VASCULITIS |
|
Infectious diseases |
|
Bacterial endocarditis |
|
Disseminated gonococcal infection |
|
Pulmonary histoplasmosis |
|
Coccidioidomycosis |
|
Syphilis |
|
Lyme disease |
|
Rocky Mountain spotted fever |
|
Whipple’s disease |
|
Coagulopathies/thrombotic microangiopathies |
|
Antiphospholipid antibody syndrome |
|
Thrombotic thrombocytopenic purpura |
|
Neoplasms |
|
Atrial myxoma |
|
Lymphoma |
|
Carcinomatosis |
|
Drug toxicity |
|
Cocaine |
|
Amphetamines |
|
Ergot alkaloids |
|
Methysergide |
|
Arsenic |
|
Sarcoidosis |
|
Atheroembolic disease |
|
Goodpasture’s syndrome |
|
Amyloidosis |
|
Migraine |
|
Cryofibrinogenemia |
The constellation of clinical, laboratory, biopsy, and radiographic findings usually allows proper categorization to a specific syndrome, and therapy where appropriate should be initiated according to this information (see individual syndromes below). If an offending antigen that precipitates the vasculitis is recognized, the antigen should be removed where possible. If the vasculitis is associated with an underlying disease such as an infection, neoplasm, or connective tissue disease, the underlying disease should be treated. If the syndrome does not resolve following removal of an offending antigen or treatment of an underlying disease, or if there is no recognizable underlying disease, treatment should be initiated according to the category of the vasculitis syndrome. Treatment options will be considered under the individual syndromes (see below), and general principles of therapy will be considered at the end of the topic.
FIGURE 10-1
Algorithm for the approach to a patient with suspected diagnosis of vasculitis. PAN, polyarteritis nodosa.