Definition
Systemic sclerosis (SSc) is a chronic systemic disorder of unknown etiology. SSc is characterized by thickening of the skin (scleroderma) and distinctive involvement of multiple internal organs, most notably the lungs, gastrointestinal tract, heart, and kidneys. The early stage of the disease, associated with prominent inflammatory features, is followed by the development of widespread functional and structural alterations in multiple vascular beds and progressive visceral organ dysfunction due to fibrosis. The presence of thickened skin (scleroderma) distinguishes SSc from other connective tissue diseases. Scleroderma-like skin induration can occur in various disorders in addition to localized forms of scleroderma (Table 7-1), and it is important to accurately differentiate these conditions from SSc. The disease is highly heterogeneous. Patients with SSc can be classified into two distinct subsets defined by the distribution pattern and extent of skin involvement, as well as other clinical and laboratory manifestations (Table 7-2). Diffuse cutaneous SSc (dcSSc) presents with progressive skin induration, starting in the fingers and ascending from distal to proximal extremities, the face, and the trunk. These patients are at risk for early pulmonary fibrosis and acute renal involvement. In limited cutaneous SSc (lcSSc), patients generally have long-standing Raynaud’s phenomenon before other manifestations of SSc appear. Skin induration is limited to the fingers (sclerodactyly), distal extremities, and face, and the trunk is not affected. A subset of patients with lcSSc have prominent calcinosis cutis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia, a constellation termed CREST syndrome. However, these features may also be seen in patients with dcSSc.Visceral organ involvement in lcSSc tends to show insidious progression. Although the long-term prognosis of lcSSc is better than that of dcSSc, pulmonary arterial hypertension (PAH), hypothyroidism, and primary biliary cirrhosis may occur in the late stage of the former. In some patients, Raynaud’s phenomenon and other typical features of SSc occur in the absence of detectable skin thickening. This syndrome has been termed SSc sine scleroderma.
Epidemiology
SSc is an acquired sporadic disease with a worldwide distribution and affecting all races. In the United States, the incidence is 9-19 cases per million per year. The only community-based survey of SSc yielded a prevalence of 286 cases per million popula-tion.There are an estimated 100,000 cases of SSc in the United States, although this number may be significantly higher if patients who do not meet strict classification criteria are also included. Studies from England, Australia, and Japan showed rates of SSc that were lower than in the United States. Age, gender, and ethnicity are important factors determining disease susceptibility. Like other connective tissue diseases, SSc shows a female predominance, greatest in the child-bearing years and declining after menopause. While SSc can present at any age, the most common age of onset is in the range of 30-50 years. African Americans have a higher incidence than whites, and disease onset occurs at an earlier age. Furthermore, African Americans are more likely to have the diffuse cutaneous form of SSc with interstitial lung involvement as well as a worse prognosis.
| TABLE 7-1 |
|
CONDITIONS ASSOCIATED WITH SCLERODERMA-LIKE INDURATION |
|
Systemic sclerosis |
|
Limited cutaneous SSc |
|
Diffuse cutaneous SSc |
|
Localized scleroderma |
|
Guttate morphea, diffuse morphea |
|
Linear scleroderma, coup de sabre, hemifacial atrophy |
|
Overlap syndromes |
|
Mixed connective tissue disease |
|
SSc/polymyositis |
|
Undifferentiated connective tissue disease |
|
Scleredema and diabetic scleredema |
|
Scleromyxedema (papular mucinosis) |
|
Nephrogenic fibrosing syndrome (nephrogenic fibrosing dermatopathy) |
|
Chronic graft-versus-host disease |
|
Diffuse fasciitis with eosinophilia (Shulman disease, eosinophilic fasciitis) |
|
Eosinophilia-myalgia syndrome |
|
Chemically induced scleroderma-like conditions |
|
Vinyl chloride-induced disease |
|
Pentazocine-induced skin fibrosis |
|
Paraneoplastic syndrome |
Genetic Considerations
A genetic contribution to disease susceptibility is indicated by the fact that 1.6% of SSc patients have a first-degree relative with SSc. The risk of other autoimmune diseases, including systemic lupus erythematosus (Chap. 4) and rheumatoid arthritis (Chap. 5), is also increased. Among Choctaw Native Americans, SSc prevalence as high as 4690 per million has been reported. Genetic investigations in SSc to date have focused on polymorphisms of candidate genes, particularly those involved in immunity and inflammation, vascular function, and connective tissue homeostasis. Associations of single nucleotide polymorphism (SNP) with SSc have been reported in the genes encoding angiotensin-converting enzyme (ACE);endothelin-1 and nitric oxide synthase;B cell markers (CD19); chemokines (monocyte chemoat-tractant protein-1) and chemokine receptors; cytokines [interleukin (IL) 1α, IL-4, and tumor necrosis factor α (TNF-α)]; growth factors and their receptors [connective tissue growth factor (CTGF) and transforming growth factor β (TGF-β)]; and extracellular matrix proteins [fibronectin, fibrillin, and secreted protein acidic-rich in cysteine (SPARC)].
Environmental Factors
Patients with SSc have increased serum antibodies to human cytomegalovirus (hCMV),and antitopoisomerase-I (Scl-70) autoantibodies recognize antigenic epitopes present on the hCMV-derived proteins, suggesting molecular mimicry as a possible mechanistic link between hCMV infection and SSc. Evidence of human parvovirus B19 infection in SSc patients has also been presented; however, the etiologic role of viruses remains unproven. Reports of apparent geographic clustering of SSc cases suggesting shared environmental exposures have not been substantiated by careful investigation.
TABLE 7-2
|
SUBSETS OF SYSTEMIC SCLEROSIS (SSc): LIMITED CUTANEOUS SSc VERSUS DIFFUSE CUTANEOUS SSc |
||
|
FEATURES |
LIMITED CUTANEOUS SSc |
DIFFUSE CUTANEOUS SSc |
|
Skin involvement |
Limited to fingers, distal to elbows, face; slow progression |
Diffuse: fingers, extremities, face, trunk; rapid progression |
|
Raynaud’s phenomenon Pulmonary fibrosis |
Precedes skin involvement; associated with critical ischemia May occur, moderate |
Onset contemporaneous with skin involvement Frequent, early and severe |
|
Pulmonary arterial hypertension |
Frequent, late, may be isolated |
May occur, associated with pulmonary fibrosis |
|
Scleroderma renal crisis Calcinosis cutis |
Very rare Frequent, prominent |
Occurs in 15%; early May occur, mild |
|
Characteristic autoantibodies |
Anticentromere |
Antitopoisomerase I (Scl-70) |
An epidemic of a novel syndrome with features suggestive of SSc occurred in Spain in the 1980s. The outbreak, termed toxic oil syndrome and affecting over 20,000 individuals, was linked to consumption of contaminated rapeseed oils used for cooking. A similar epidemic outbreak, termed eosinophilia-myalgia syndrome (EMS), occurred a decade later in the United States. Affected individuals presented with marked peripheral blood eosinophilia and severe myalgia, followed by the development of scleroderma-like chronic skin lesions. The epidemic was linked to the consumption of imported batches of l-tryptophan used as dietary supplements. The incidence rate of new cases of EMS showed a dramatic decline following the nationwide recall of L-tryptophan products. While both of these apparently novel toxic-epidemic syndromes were characterized by sclerodermalike chronic skin changes and variable visceral organ involvement, their clinical, pathological, and laboratory features clearly distinguished them from SSc. The incidence of SSc is increased among miners exposed to silica. Other occupational exposures tentatively linked with SSc include polyvinyl chloride, epoxy resins, and aromatic hydrocarbons including toluene and trichloroethylene. Drugs implicated in SSc-like illnesses include bleomycin, pentazocine and cocaine, and appetite suppressants linked with pulmonary hypertension. Case reports and series describing SSc in women with silicone breast implants had raised concern regarding a possible etiologic role of silicone in SSc. However, large-scale epidemiologic investigations found no evidence of increased risk of SSc.
Pathogenesis
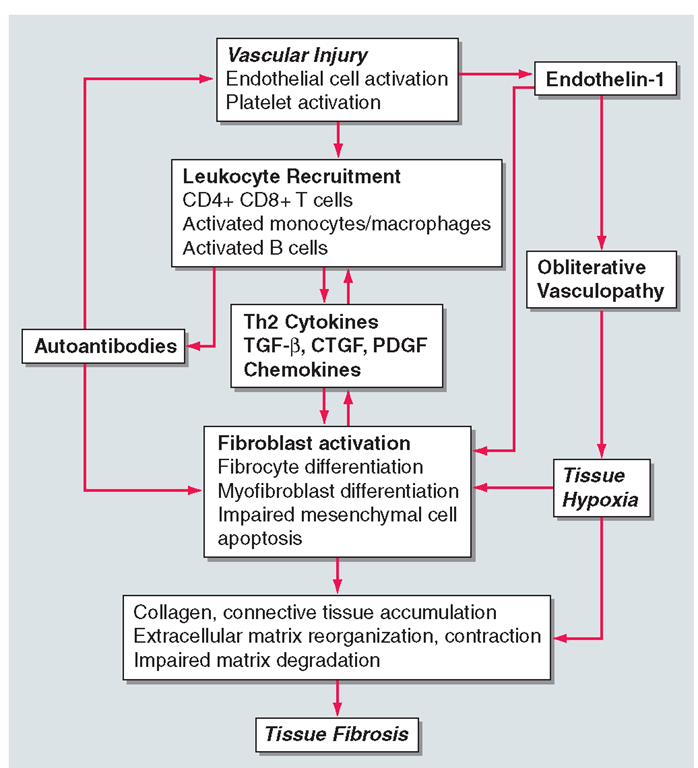
A comprehensive view of the pathogenesis of SSc must take into account the three cardinal features of the disease: (1) vasculopathy, (2) cellular and humoral immunity, and (3) progressive visceral and vascular fibrosis in multiple organs (Fig. 7-1). Autoimmunity and altered endothelial cell function and vascular reactivity may be the earliest manifestations of SSc. Complex interplay between these processes is thought to initiate and then amplify and sustain the fibrotic process.
Animal Models of Disease
There is no single animal model that reproduces all three cardinal processes that underlie the pathogenesis of SSc, but some models recapitulate selected disease characteristics. The tight-skin mouse is a naturally occurring fibrosis model characterized by spontaneous skin thickening. The mutation responsible for the phenotype, a duplication in the fibrillin-1 gene, results in defective extracellular matrix assembly and aberrant activation of TGF-β. Mutations in the fibrillin-1 gene have not been described in patients with SSc. Chronic fibrosis in the skin and lungs can be induced in mice by bleomycin injections or by transplantation of HLA-mismatched bone marrow or spleen cells. Increasingly, manipulation of mice via mutagenesis or targeted genetic modification such as knock-out or transgenesis are utilized to create new models for SSc and to dissect the roles of individual molecules in the underlying processes. For instance, genetic targeting of Smad3, an intracellular TGF-β signal transducer, or of the chemokine monocyte chemoattractant protein-1 (MCP-1) yielded mice that were resistant to bleomycin-induced scleroderma.
FIGURE 7-1
The pathogenesis of systemic sclerosis. Initial vascular injury in genetically susceptible individuals leads to functional and structural vascular alterations, inflammation, and autoimmunity. The inflammatory and immune responses initiate and sustain fibroblast activation and differentiation, resulting in pathological fibrogenesis and irreversible tissue damage.
Vasculopathy
Vascular involvement in SSc is extensive and has important clinical consequences. Raynaud’s phenomenon, an early manifestation, is characterized by an altered blood-flow response to cold challenge. This initially reversible vascular abnormality is associated with alterations in the autonomic and peripheral nervous systems, with impaired production of neuropeptides such as calcitonin gene-related peptide from sensory afferent nerves and heightened sensitivity of a2-adrenergic receptors on vascular smooth-muscle cells.While isolated Raynaud’s phenomenon is relatively benign and nonprogressive, in patients with SSc it is associated with irreversible morphological and functional changes. Viruses, superoxide radicals, and vascular cytotoxic factors such as serum proteases, complement, and circulating antiendothelial cell autoantibodies may each contribute to endothelial cell injury in early SSc. Endothelial injury results in dysregulated production of endothelium-derived vasodilatory (nitric oxide and prostacyclin) and vasoconstricting (endothelin-1) substances, as well as increased expression of intercellular adhesion molecule 1 (ICAM-1) and other surface adhesion molecules. Microvessels show enhanced permeability and transendothelial leukocyte diapedesis, activation of coagulation and fibrinolytic cascades, and platelet aggregation. Smooth-muscle cell-like myointimal cells proliferate, the basement membrane is thickened and reduplicated, and fibrosis of the adventitial layers develops. The vasculopathic process affects capillaries, arterioles, and even large vessels in many organs, resulting in reduced blood flow and impaired tissue oxygenation.
Progressive luminal occlusion due to intimal and medial hypertrophy and adventitial fibrosis, combined with persistent endothelial cell damage and apoptosis, establish a vicious cycle culminating in the striking absence of blood vessels seen on angiograms of the hands and kidneys of patients with late-stage disease. Damaged endothelium promotes platelet aggregation with release of platelet alpha granules including thromboxane, a potent vasoconstrictor, and of platelet-derived growth factor (PDGF).Vascular compromise is further aggravated by defective fibrinolysis. Oxidative stress due to ischemia-reperfusion is associated with generation of free radicals that further damage the endothelium through peroxidation of membrane lipids. Paradoxically, the process of revascularization that normally reestablishes blood flow to ischemic tissue is defective in SSc. Failure of vasculogenesis occurs in the setting of elevated serum levels of vascular endothelial growth factor (VEGF) and other angiogenic factors. The number of bone marrow-derived CD34+ CD133+ endothelial progenitor cells is markedly reduced in the circulation, and their differentiation in vitro into mature endothelial cells is impaired. Thus, widespread obliterative vasculopathy and failure to repair damaged vessels are hallmarks of SSc.
Cellular and Humoral Autoimmunity
In the early stages of SSc, activated T cells and monocytes/ macrophages accumulate in lesional skin, lungs, and other affected organs. Infiltrating T cells express CD45 and HLA-DR activation markers and display restricted receptor signatures indicative of oligoclonal expansion in response to (unknown) antigen. Circulating CD4+ T cells have elevated levels of chemokine receptors and α inte-grin adhesion molecules accounting for their enhanced ability to bind to endothelium and to fibroblasts. Endothelial cells express ICAM-1 and other adhesion molecules that facilitate leukocyte diapedesis. Activated macrophages and T cells show a TH2-polarized immune response and secrete IL-4 and IL-13. TH2 cytokines induce the production of TGF-β and promote collagen synthesis and other profibrotic responses, whereas the TH1 cytokine interferon γ (IFN-γ) inhibits collagen synthesis and blocks cytokine-mediated fibroblast activation. Because TGF-β stimulates its own synthesis, as well as that of CTGF (also termed CCN2) and other cytokines,TGF-ß establishes an autocrine/paracrine stimulatory loop that sustains activation of fibroblasts and other effector cells (Chaps. 1 and 3).
Circulating autoantibodies occur in virtually all patients with SSc. These mutually exclusive autoantibodies are highly specific for SSc and show strong association with individual disease phenotypes and genetically determined HLA haplotypes. Autoantibody levels correlate with disease severity, and titers fluctuate with disease activity. Some SSc-specific autoantibodies are antinuclear and directed against proteins involved in mitosis, such as topoisomerase-I, centromere, and the RNA polymerases; others are directed against cell-surface antigens or secreted proteins. While autoantibodies have well-established clinical utility as diagnostic and prognostic markers, their pathogenetic role in the clinical manifestations of SSc remains uncertain. Topoisomerase-I autoantibodies can directly bind to fibroblasts, and autoantibodies to fibroblasts, endothelial cells, PDGF cell-surface receptors, fibrillin-1, and matrix metalloproteinase enzymes have been described in SSc patients. Some of these autoantibodies may play a direct role in tissue damage.
Multiple potential mechanisms have been proposed to account for the generation of autoantibodies in SSc. According to one theory, in patients with SSc, certain proteins undergo modifications such as proteolytic cleavage, increased expression, or altered subcellular localization, resulting in their recognition by the immune system. For example, the protease granzyme B is released from cytotoxic T cells, cleaves peptides, and generates potential neoepitopes that can break immune tolerance. Recent studies implicate B cells in both the autoimmune and fibrotic responses in SSc. In addition to their well-recognized role in antibody production, B cells can also present antigen, produce IL-6 and TGF-ß, and modulate T cell and dendritic cell function. In patients with SSc, B cells show elevated expression of the CD19 membrane receptor, the naive B cell compartment is expanded, and numbers of memory B cells and early plasma cells are reduced. Gene expression profiling of lesional skin has identified mRNA expression signatures characteristic of activated B cells.
Fibrosis
Fibrosis affecting multiple organs distinguishes SSc from other connective tissue diseases. Fibrosis characteristically follows and is thought to be a consequence of autoimmunity and vascular damage. The process, characterized by progressive replacement of normal tissue architecture with dense connective tissue, accounts for substantial morbidity and mortality in SSc. Fibroblasts are mesenchymal cells responsible for maintaining the functional and structural integrity of connective tissue.When activated by TGF-β and related factors, fibroblasts proliferate, migrate, elaborate collagen and extracellular matrix, secrete growth factors and cytokines and express surface receptors for them, and transdifferentiate into myofibroblasts. Together, these responses allow fibroblasts to repair tissue injury under normal circumstances. While the fibroblast repair program is rapid and self-limited under physiologic conditions, the activation of fibroblasts in pathological fibrosis is sustained and amplified, resulting in exaggerated matrix remodeling and scar formation.
In addition to resident connective tissue fibroblasts, circulating mesenchymal progenitor cells of bone marrow origin also contribute to fibrosis. The factors that regulate the production of mesenchymal progenitor cells in the bone marrow and their trafficking from the circulation into lesional tissue, and promote their differentiation in situ into matrix-producing adhesive and contractile fibro-cytes, are unknown. Fibroblasts can undergo transdifferentiation into smooth-muscle-like myofibroblasts. While myofibroblasts can be transiently detected during normal wound healing, they persist in tissue during pathological fibrogenesis, possibly due to abnormal resistance to apop-tosis. Myofibroblasts contribute to scar formation via their ability to produce collagen and TGF-β and to contract the surrounding extracellular matrix, converting it into dense scar.
Fibroblasts explanted from SSc lesional tissues display an abnormal phenotype in culture indicative of autonomous activation. Compared to normal fibroblasts, SSc fibroblasts are characterized by variably increased rates of collagen gene transcription. Furthermore, they display smooth-muscle actin stress fibers; enhanced synthesis and secretion of extracellular matrix molecules, cytokines, and growth factors; expression of chemokine receptors and cell surface adhesion molecules; resistance to apoptosis; and constitutive autocrine TGF-β signaling. The abnormal “scleroderma phenotype” persists during serial passage of these cells in vitro. The mechanisms underlying the autonomously activated phenotype are unknown; persistent fibroblast activation via autocrine stimulatory loops involving TGF-β, selection of activated fibroblast subpopulations driven by hypoxia or immune factors, intrinsic abnormalities in SSc fibroblasts, and altered cell-matrix interaction may be involved. Recent studies indicate that intracellular blockade of TGF-β signaling can partially “normalize” the activated phenotype in SSc lesional fibroblasts, suggesting that autocrine TGF-β signaling contributes to the persistent fibrogenic phenotype of SSc fibroblasts. Results from global transcriptome analyses of SSc fibroblasts show differential expression of many extracellular matrix genes, including collagens, fibronectin, and fibrillins. A majority of the abnormally expressed genes could be mechanistically linked to TGF-β responses, but other fibrogenic signaling pathways also operate in SSc.