Definition and Prevalence
Systemic lupus erythematosus (SLE) is an autoimmune disease in which organs and cells undergo damage mediated by tissue-binding autoantibodies and immune complexes. Ninety percent of patients are women of child-bearing years; people of both sexes, all ages, and all ethnic groups are susceptible. Prevalence of SLE in the United States is 15-50 per 100,000; the highest prevalence among ethnic groups studied is in African Americans.
Pathogenesis and Etiology
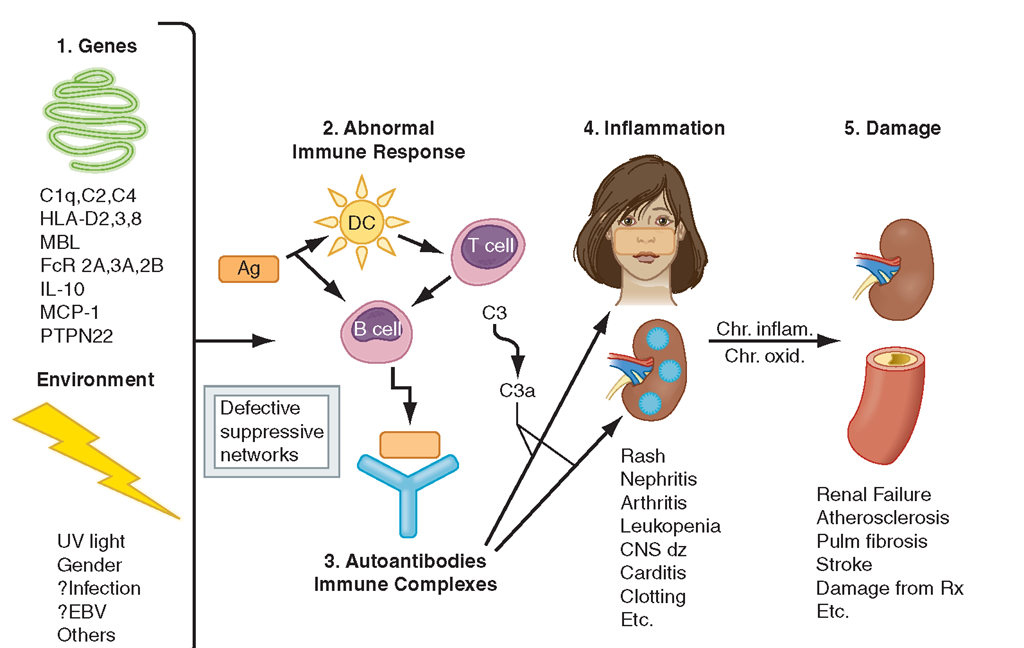
The proposed pathogenic mechanisms of SLE are illustrated in Fig. 4-1. Interactions between susceptibility genes and environmental factors result in abnormal immune responses.Those responses include (1) activation of innate immunity (dendritic cells) by CpG DNA, DNA in immune complexes, and RNA in RNA/protein selfantigens; (2) lowered activation thresholds of adaptive immunity cells (antigen-specific T and B lymphocytes); (3) ineffective regulatory and inhibitory CD4+ and CD8+ T cells; and (4) reduced clearance of apoptotic cells and of immune complexes. Self-antigens (nucleosomal DNA/ protein; RNA/protein in Sm, Ro, and La; phospholipids) are available for recognition by the immune system in surface blebs of apoptotic cells; thus antigens, autoantibodies, and immune complexes persist for prolonged periods of time, allowing inflammation and disease to develop. Immune activation of circulating and tissue-bound cells is accompanied by increased secretion of proinflammatory tumor necrosis factor (TNF) α and type 1 and 2 interferons (IFNs), and the B cell-driving cytokines B lymphocyte stimulator (BLyS) and interleukin (IL)-10. Upregulation of genes induced by interferons is a genetic “signature” of SLE. However, lupus T and natural killer (NK) cells fail to produce enough IL-2 and transforming growth factor (TGF) to induce regulatory CD4+ and inhibitory CD8+ T cells.The result ofthese abnormalities is sustained production of pathogenic autoantibodies (referred to in Fig. 4-1 and described in Table 4-1) and immune complexes, which bind to target tissues, with activation of complement and phagocytic cells that recognize Ig-coated circulating blood cells. Activation of complement and immune cells leads to release of chemotaxins, cytokines, chemokines, vasoactive peptides, and destructive enzymes. In the setting of chronic inflammation, accumulation of growth factors and products of chronic oxidation contribute to irreversible tissue damage in glomeruli, arteries, lungs, and other tissues.
SLE is a multigenic disease. In most genetically susceptible individuals, normal alleles of multiple normal genes each contribute a small amount to abnormal immune responses; if enough variations accumulate, disease results. Some predisposing genes confirmed in at least two independent cohorts are listed in Fig. 4-1. Homozygous deficiencies of early components of complement (C1q,r,s; C2; C4) confer strong predisposition to SLE, but such deficiencies are rare.
FIGURE 4-1
Pathogenesis of SLE. Genes confirmed in more than one independent cohort as increasing susceptibility to SLE or lupus nephritis are listed. Gene-environment interactions result in abnormal immune responses that generate pathogenic autoantibodies and immune complexes that deposit in tissue, activate complement, cause inflammation, and over time lead to irreversible organ damage. Ag, antigen; C1q, complement system; C3, complement component; CNS, central nervous system; DC, dendritic cell; EBV, Epstein-Barr virus; HLA, human leukocyte antigen; FcR, immunoglobulin Fc-binding receptor; IL, interleukin; MBL, mannose-binding ligand; MCP, monocyte chemotactic protein; PTPN, phos-photyrosine phosphatase; UV, ultraviolet.
Each of the other genes listed increases risk for SLE by only 1.5- to 3-fold. Some gene alleles probably contribute to disease susceptibility by influencing clearance of apoptotic cells (C1q, MBL) or immune complexes (FcR 2A and 3A), antigen presentation (HLA-DR2,3,8),B cell maturation (IL-10),T cell activation (PTPN22), or chemotaxis (MCP-1). None of these hypotheses is proven. In addition to influencing disease susceptibility in various ethnic groups, some genes influence clinical manifestations of disease (e.g., FcR 2A/3A, MBL, PDCD1 for nephritis; MCP-1 for arthritis and vasculitis). A region on chromosome 16 contains genes that predispose to SLE, rheumatoid arthritis, psoriasis, and Crohn’s disease, suggesting the presence of“autoimmunity genes” that, when interacting with other genes, predispose to different autoimmune diseases. There are likely to be protective gene alleles as well. All these gene combinations influence immune responses to the external and internal environment; when such responses are too high and/or too prolonged, autoimmune disease results.
Female sex is permissive for SLE; females of many mammalian species make higher antibody responses than males.Women exposed to estrogen-containing oral contraceptives or hormone replacements have an increased risk of developing SLE (1.2- to 2-fold). Estradiol binds to receptors on T and B lymphocytes, increasing activation and survival of those cells, thus favoring prolonged immune responses.
Several environmental stimuli may influence SLE (Fig. 4-1). Exposure to ultraviolet light causes flares of SLE in approximately 70% of patients, possibly by increasing apoptosis in skin cells or by altering DNA and intracellular proteins to make them antigenic. It is likely that some infections induce a normal immune response that matures to contain some T and B cells that recognize self-antigens; such cells are not appropriately regulated, and autoantibody production occurs. Most SLE patients have autoantibodies for 3 years or more before the first symptoms of disease, suggesting that regulation controls the degree of autoimmunity for years before quantities and qualities of autoantibodies and pathogenic B and T cells actually cause clinical disease. Epstein-Barr virus (EBV) may be one infectious agent that can trigger SLE in susceptible individuals. Children and adults with SLE are more likely to be infected by EBV than age-, sex-, and ethnicity-matched controls—an observation confirmed in African-American adults in another population. EBV activates and infects B lymphocytes and survives in those cells for decades; it also contains amino acid sequences that mimic sequences on human spliceosomes (RNA/protein antigens often recognized by autoantibodies in people with SLE). Thus, interplay between genetic susceptibility, environment, sex, and abnormal immune responses results in autoimmunity.
TABLE 4-1
|
AUTOANTIBODIES IN SYSTEMIC LUPUS ERYTHEMATOSUS (SLE) |
|||
|
ANTIBODY |
PREVALENCE, % |
ANTIGEN RECOGNIZED |
CLINICAL UTILITY |
|
Antinuclear antibodies |
98 |
Multiple nuclear |
Best screening test; repeated negative tests make SLE unlikely |
|
Anti-dsDNA |
70 |
DNA (double-stranded) |
High titers are SLE specific and in some patients correlate with disease activity, nephritis, vasculitis |
|
Anti-Sm |
25 |
Protein complexed to 6 species of nuclear U1 RNA |
Specific for SLE; no definite clinical correlations; most patients also have anti-RNP; more common in African Americans and Asians than Caucasians |
|
Anti-RNP |
40 |
Protein complexed to U1 RNAγ |
Not specific for SLE; high titers associated with syndromes that have overlap features of several rheumatic syndromes including SLE; more common in African Americans than Caucasians |
|
Anti-Ro (SS-A) |
30 |
Protein complexed to hY RNA, primarily 60 kDa and 52 kDa |
Not specific for SLE; associated with sicca syndrome, subacute cutaneous lupus, and neonatal lupus with congenital heart block; associated with decreased risk for nephritis |
|
Anti-La (SS-B) |
10 |
47-kDa protein complexed to hY RNA |
Usually associated with anti-Ro; associated with decreased risk for nephritis |
|
Antihistone |
70 |
Histones associated with DNA (in nucleosome, chromatin) |
More frequent in drug-induced lupus than in SLE |
|
Antiphospholipid |
50 |
Phospholipids, β2 glycoprotein 1 cofactor, prothrombin |
Three tests available—ELISAs for cardiolipin and ß2G1, sensitive prothrombin time (DRVVT); predisposes to clotting, fetal loss, thrombocytopenia |
|
Antierythrocyte |
60 |
Erythrocyte membrane |
Measured as direct Coombs’ test; a small proportion develops overt hemolysis |
|
Antiplatelet |
30 |
Surface and altered cytoplasmic antigens in platelets |
Associated with thrombocytopenia but sensitivity and specificity are not good; this is not a useful clinical test |
|
Antineuronal (includes antiglutamate receptor) |
60 |
Neuronal and lymphocyte surface antigens |
In some series a positive test in CSF correlates with active CNS lupus |
|
Antiribosomal P |
20 |
Protein in ribosomes |
In some series a positive test in serum correlates with depression or psychosis due to CNS lupus |
Note: CNS, central nervous system; CSF, cerebrospinal fluid; DRVVT, dilute Russell viper venom time; ELISA, enzyme-linked immunosorbent assay.
Pathology
In SLE, biopsies of affected skin show deposition of Ig at the dermal-epidermal junction (DEJ), injury to basal ker-atinocytes, and inflammation dominated by T lymphocytes in the DEJ and around blood vessels and dermal appendages. Clinically unaffected skin may also show Ig deposition at the DEJ.
In renal biopsies, the pattern and severity of injury are important in diagnosis and in selecting the best therapy. Most published clinical studies of lupus nephritis have used the World Health Organization (WHO) classification of lupus nephritis. However, the International Society of Nephrology (ISN) and the Renal Pathology Society (RPS) have published a new, similar classification (Table 4-2) that will probably replace the WHO standards. An advantage of the ISN/RPS classification is the addition of “a” for active and “c” for chronic changes, giving the physician information regarding the potential reversibility of disease. All the classification systems focus on glomerular disease, although the presence of tubular interstitial and vascular disease is important to clinical outcomes.
TABLE 4-2
|
CLASSIFICATION OF LUPUS NEPHRITIS (INTERNATIONAL SOCIETY OF NEPHROLOGY AND RENAL PATHOLOGY SOCIETY) |
|
Class I: Minimal Mesangial Lupus Nephritis |
|
Normal glomeruli by light microscopy, but mesangial immune deposits by immunofluorescence. |
|
Class II: Mesangial Proliferative Lupus Nephritis |
|
Purely mesangial hypercellularity of any degree or mesangial matrix expansion by light microscopy, with mesangial immune deposits. A few isolated subepithelial or subendothelial deposits may be visible by immunofluorescence or electron microscopy, but not by light microscopy. |
|
Class III: Focal Lupus Nephritis |
|
Active or inactive focal, segmental or global endo- or extracapillary glomerulonephritis involving <50% of all glomeruli, typically with focal subendothelial immune deposits, with or without mesangial alterations. Class III (A): Active lesions—focal proliferative lupus nephritis Class III (A/C): Active and chronic lesions—focal proliferative and sclerosing lupus nephritis Class III (C): Chronic inactive lesions with glomerular scars—focal sclerosing lupus nephritis |
|
Class IV: Diffuse Lupus Nephritis |
|
Active or inactive diffuse, segmental, or global endo- or extracapillary glomerulonephritis involving 50% of all glomeruli, typically with diffuse subendothelial immune deposits, with or without mesangial alterations. This class is divided into diffuse segmental (IV-S) lupus nephritis when 50% of the involved glomeruli have segmental lesions, and diffuse global (IV-G) lupus nephritis when 50% of the involved glomeruli have global lesions. Segmental is defined as a glomerular lesion that involves less than half of the glomerular tuft. This class includes cases with diffuse wire loop deposits but with little or no glomerular proliferation. |
|
Class IV-S (A): Active lesions—diffuse segmental proliferative lupus nephritis |
|
Class IV-G (A): Active lesions — diffuse global proliferative lupus nephritis |
|
Class IV-S (A/C): Active and chronic lesions — diffuse segmental proliferative and sclerosing lupus nephritis |
|
Class IV-G (A/C): Active and chronic lesions—diffuse global proliferative and sclerosing lupus nephritis |
|
Class IV-S (C): Chronic inactive lesions with scars — diffuse segmental sclerosing lupus nephritis |
|
Class IV-G (C): Chronic inactive lesions with scars—diffuse global sclerosing lupus nephritis |
|
Class V: Membranous Lupus Nephritis |
|
Global or segmental subepithelial immune deposits or their morphologic sequelae by light microscopy and by immunofluo rescence or electron microscopy, with or without mesangial alterations. Class V lupus nephritis may occur in combination with class III or IV, in which case both will be diagnosed. Class V lupus nephritis may show advanced sclerosis. |
|
Class VI: Advanced Sclerotic Lupus Nephritis |
|
>90% of glomeruli globally sclerosed without residual activity. |
Indicate and grade (mild, moderate, severe) tubular atrophy, interstitial inflammation and fibrosis, severity of arteriosclerosis or other vascular lesions.
In general, class III and IV disease, as well as class V accompanied by III or IV disease, should be treated with aggressive immunosuppression if possible, because there is a high risk for end-stage renal disease (ESRD) if patients are untreated or undertreated. Treatment for lupus nephritis is not recommended in patients with class I or II disease or with extensive irreversible changes. In children, a diagnosis of SLE can be established on the basis of renal histology without meeting additional diagnostic criteria (Table 4-3).
Histologic abnormalities in blood vessels may also determine therapy. Patterns of vasculitis are not specific for SLE but may indicate active disease: leukocytoclastic vasculitis is most common.
Lymph node biopsies are usually performed to rule out infection or malignancies. In SLE, they show nonspecific diffuse chronic inflammation.