Clinical Manifestations
CPPD arthropathy may be asymptomatic, acute, subacute, or chronic or cause acute synovitis superimposed on chronically involved joints. Acute CPPD arthritis was originally termed pseudogout by McCarty and coworkers because of its striking similarity to gout. Other clinical manifestations of CPPD deposition include (1) induction or enhancement of peculiar forms of osteoarthritis; (2) induction of severe destructive disease that may radiographically mimic neuropathic arthritis; (3) production of symmetric proliferative synovitis, clinically similar to rheumatoid arthritis and frequently seen in familial forms with early onset; (4) intervertebral disk and ligament calcification with restriction of spine mobility, mimicking ankylosing spondylitis (also seen in hereditary forms); and (5) rarely spinal stenosis (most commonly seen in the elderly) (Table 19-1).
The knee is the joint most frequently affected in CPPD arthropathy. Other sites include the wrist, shoulder, ankle, elbow, and hands. Rarely, the temporomandibular joint and ligamentum flavum of the spinal canal are involved. Clinical and radiographic evidence indicates that CPPD deposition is polyarticular in at least two-thirds of patients. When the clinical picture resembles that of slowly progressive osteoarthritis, diagnosis may be difficult. Joint distribution may provide important clues suggesting CPPD disease. For example, primary osteoarthritis rarely involves a metacarpophalangeal, wrist, elbow, shoulder, or ankle joint. If radiographs reveal punctate and/or linear radiodense deposits in fibrocartilaginous joint menisci or articular hyaline cartilage (chondrocalcinosis), the diagnostic likelihood of CPPD disease is further enhanced. Definitive diagnosis requires demonstration of typical crystals in synovial fluid or articular tissue (Fig. 19-2). In the absence of joint effusion or indications to obtain a synovial biopsy, chondrocalcinosis is presumptive of CPPD deposition. One exception is chondrocalcinosis due to CaOx in some patients with chronic renal failure.
Acute attacks of CPPD arthritis may be precipitated by trauma. Rapid diminution of serum calcium concentration, as may occur in severe medical illness or after surgery (especially parathyroidectomy), can also lead to pseudogout attacks.
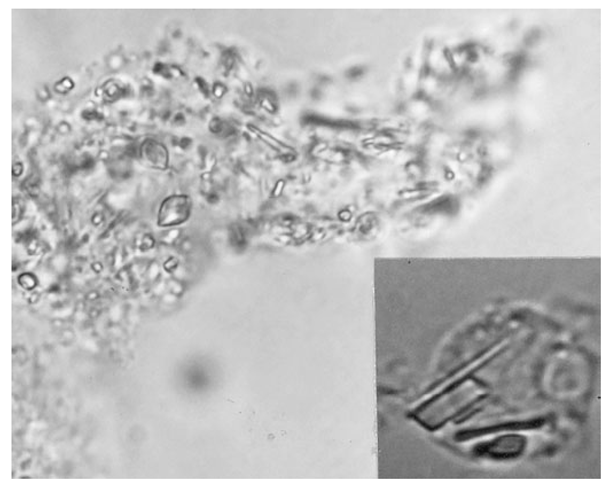
FIGURE 19-2
Intracellular and extracellular calcium pyrophosphate dihydrate crystals, as seen in a fresh preparation of synovial fluid, illustrate rectangular, rod-shaped, and rhomboid weakly positive birefringent crystals (compensated polarized light microscopy; 400x).
In as many as 50% of cases, episodes of CPPD-induced inflammation are associated with low-grade fever and, on occasion, temperatures as high as 40°C. Whether or not radiographic proof of chondrocalcinosis is evident in the involved joint(s), synovial fluid analysis with microbial cultures is essential to rule out the possibility of infection. In fact, infection in a joint with any microcrystalline deposition process can lead to crystal shedding and subsequent synovitis from both crystals and microorganisms. Synovial fluid in acute CPPD disease has inflammatory characteristics. The leukocyte count can range from several thousand cells to 100,000 cells^L, the mean being about 24,000 cells^L and the predominant cell being the neutrophil. Polarized light microscopy usually reveals rhomboid, square, or rod-like crystals with weak positive birefringence inside tissue fragments and fibrin clots and in neutrophils (Fig. 19-2). CPPD crystals may coexist with MSU and apatite in some cases.
Treatment:
CPPD Deposition Disease
Untreated acute attacks may last a few days to as long as a month. Treatment by joint aspiration and NSAIDs or by intraarticular glucocorticoid injection may result in return to prior status in <10 days. For patients with frequent recurrent attacks of pseudogout, daily prophylactic treatment with low doses of colchicine may be helpful in decreasing the frequency of the attacks. Severe polyarticular attacks usually require short courses of glucocorticoids. Unfortunately, there is no effective way to remove CPPD deposits from cartilage and synovium. Uncontrolled studies suggest that the administration of antimalarial agents or even methotrexate may be helpful in controlling persistent synovitis. Patients with progressive destructive large-joint arthropathy may require joint replacement.
Calcium Apatite Deposition Disease
Pathogenesis
Apatite is the primary mineral of normal bone and teeth. Abnormal accumulation can occur in areas of tissue damage (dystrophic calcification), in hypercalcemic or hyperparathyroid states (metastatic calcification), and in certain conditions of unknown cause (Table 19-3). In chronic renal failure, hyperphosphatemia can contribute to extensive apatite deposition both in and around joints. Familial aggregation is rarely seen; no association with ANKH mutations has been described thus far. Apatite crystals are deposited primarily on matrix vessels.
TABLE 19-3
|
CONDITIONS ASSOCIATED WITH APATITE DEPOSITION DISEASE |
|
Aging |
|
Osteoarthritis |
|
Hemorrhagic shoulder effusions in the elderly (Milwaukee shoulder) |
|
Destructive arthropathy |
|
Tendinitis, bursitis |
|
Tumoral calcinosis (sporadic cases) |
|
Disease-associated |
|
Hyperparathyroidism |
|
Milk-alkali syndrome |
|
Renal failure/long-term dialysis |
|
Connective tissue diseases (e.g., systemic sclerosis, idiopathic myositis, SLE) |
|
Heterotopic calcification following neurologic catastrophes (e.g., stroke, spinal cord injury) |
|
Heredity |
|
Bursitis, arthritis |
|
Tumoral calcinosis |
|
Fibrodysplasia ossificans progressiva |
Note: SLE, systemic lupus erythematosus.
Incompletely understood alterations in matrix proteoglycans, phosphatases, hormones, and cytokines can probably influence crystal formation.
Apatite aggregates are commonly present in synovial fluid in an extremely destructive chronic arthropathy of the elderly that occurs most often in shoulders (Milwaukee shoulder) and in a similar process in hips, knees, and erosive osteoarthritis of fingers. Joint destruction is associated with damage to cartilage and supporting structures, leading to instability and deformity. Progression tends to be indolent, and synovial fluid leukocyte counts are usually <2000^L. Symptoms range from minimal to severe pain and disability that may lead to joint replacement surgery. Whether severely affected patients merely represent an extreme synovial tissue response to the apatite crystals that are so common in osteoarthritis is uncertain. Synovial lining cell cultures exposed to apatite (or CPPD) crystals markedly increase the release of collagenases and neutral proteases, underscoring the destructive potential of abnormally stimulated synovial lining cells.
Clinical Manifestations
Periarticular or articular deposits may occur and may be associated with acute reversible inflammation and/or chronic damage to the joint capsule, tendons, bursa, or articular surfaces. The most common sites of apatite deposition include bursae and tendons in and/or around the knees, shoulders, hips, and fingers. Clinical manifestations include asymptomatic radiographic abnormalities, acute synovitis, bursitis, tendinitis, and chronic destructive arthropathy. Although the true incidence of apatite arthritis is not known, 30-50% of patients with osteoarthritis have apatite microcrystals in their synovial fluid. Such crystals can frequently be identified in clinically stable osteoarthritic joints, but they are more likely to come to attention in persons experiencing acute or subacute worsening of joint pain and swelling. The synovial fluid leukocyte count in apatite arthritis is usually low (<2000^L), despite dramatic symptoms, with predominance of mononuclear cells.
Diagnosis
Intra- and/or periarticular calcifications with or without erosive, destructive, or hypertrophic changes may be seen on radiographs (Fig. 19-3).These should be distinguished from the linear calcifications typical of CPPD deposition disease.
Definitive diagnosis of apatite arthropathy depends on identification of crystals from synovial fluid or tissue (Fig. 19-3). Individual crystals, which generally contain mostly carbonate substituted apatite, are very small and can be seen only by electron microscopy. Clumps of crystals may appear as 1- to 20^m shiny intra- or extracellular non-birefringent globules or aggregates that stain purplish with Wright’s stain and bright red with alizarin red S. Absolute identification depends on electron microscopy with energy-dispersive elemental analysis, x-ray diffraction, or infrared spectroscopy, but these are usually not required in clinical diagnosis.
Treatment:
Calcium Apatite Deposition Disease
Treatment of apatite arthritis or periarthritis is nonspecific. Acute attacks of bursitis or synovitis may be self-limiting, resolving in days to several weeks. Aspiration of effusions and the use of either NSAIDs or oral colchicine for 2 weeks or intra- or periarticular injection of a depot glucocorticoid appear to shorten the duration and intensity of symptoms. Periarticular apatite deposits may be resorbed with resolution of attacks. Agents to lower serum phosphate levels may lead to resorption of deposits in renal failure patients receiving hemodialysis. In patients with underlying severe destructive articular changes, response to medical therapy is usually less rewarding.
CaOx Deposition Disease
Pathogenesis
Primary oxalosis is a rare hereditary metabolic disorder. Enhanced production of oxalic acid may result from at least two different enzyme defects, leading to hyperox-alemia and deposition of calcium oxalate crystals in have been documented in fingers, wrists, elbows, knees, ankles, and feet.
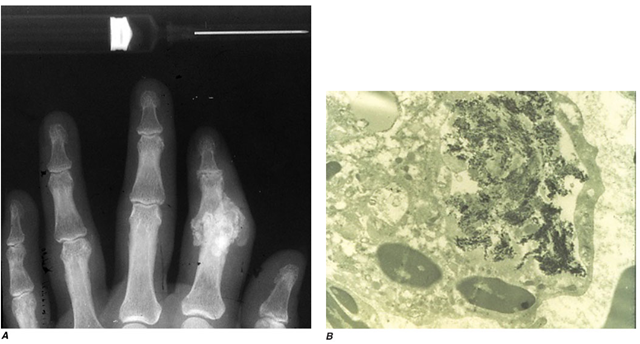
FIGURE 19-3
A. Radiograph showing calcification due to apatite crystals surrounding an eroded joint. B. An electron micrograph tissues. Nephrocalcinosis, renal failure, and death usually occur before age 20. Acute and/or chronic CaOx arthritis and periarthritis may complicate primary oxalosis during later years of illness.
Secondary oxalosis is more common than the primary disorder. It is one of the many metabolic abnormalities that complicate end-stage renal disease. In chronic renal disease, calcium oxalate deposits have long been recognized in visceral organs, blood vessels, bones, and even cartilage. However, it was not until 1982 that such deposits were demonstrated to be one of the causes of arthritis in chronic renal failure. Thus far, reported patients have been dependent on long-term hemodialysis or peritoneal dialysis, and many had received ascorbic acid supplements. Ascorbic acid is metabolized to oxalate, which is inadequately cleared in uremia and by dialysis. Such supplements are now usually avoided in dialysis programs because of the risk of enhancing hyperoxalosis and its sequelae.
Clinical Manifestations and Diagnosis
CaOx aggregates can be found in bone, articular cartilage, synovium, and periarticular tissues. From these sites, crystals may be shed, causing acute synovitis. Persistent aggregates of CaOx can, like apatite and CPPD, stimulate synovial cell proliferation and enzyme release, resulting in progressive articular destruction. Deposits demonstrates dark needle-shaped apatite crystals within a vacuole of a synovial fluid mononuclear cell (30,000x).
Clinical features of acute CaOx arthritis may not be distinguishable from those due to sodium urate, CPPD, or apatite. Radiographs may reveal chondrocalcinosis or soft-tissue calcifications. CaOx-induced synovial effusions are usually noninflammatory, with <2000 leukocytes^L, or mildly inflammatory. Neutrophils or mononuclear cells can predominate. CaOx crystals have a variable shape and variable birefringence to polarized light. The most easily recognized forms are bipyramidal, have strong birefringence (Fig. 19-4), and stain with alizarin red S.
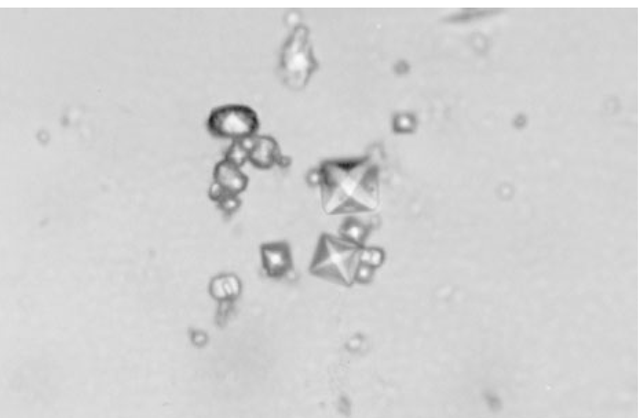
FIGURE 19-4
Bipyramidal and small polymorphic calcium oxalate crystals from synovial fluid are classical finding in CaOx arthropathy (ordinary light microscopy; 400x).
Treatment:
Calcium Oxalate Deposition Disease
Treatment of CaOx arthropathy with NSAIDs,colchicine, intraarticular glucocorticoids, and/or an increased frequency of dialysis has produced only slight improvement. In primary oxalosis, liver transplantation has induced a significant reduction in crystal deposits.