Laboratory Investigations
The vast majority of musculoskeletal disorders can be easily diagnosed by a complete history and physical examination. An additional objective of the initial encounter is to determine whether additional investigations or immediate therapy are required. A number of features indicate the need for additional evaluation. Monarticular conditions require additional evaluation, as do traumatic or inflammatory conditions and conditions accompanied by neurologic changes or systemic manifestations of serious disease. Finally, individuals with chronic symptoms (>6 weeks), especially when there has been a lack of response to symptomatic measures, are candidates for additional evaluation. The extent and nature of the additional investigation should be dictated by the clinical features and suspected pathologic process. Laboratory tests should be used to confirm a specific clinical diagnosis and not be used to screen or evaluate patients with vague rheumatic complaints. Indiscriminate use of broad batteries of diagnostic tests and radiographic procedures is rarely a useful or cost-effective means to establish a diagnosis.
Besides a complete blood count, including a white blood cell (WBC) and differential count, the routine evaluation should include a determination of an acute-phase reactant such as the ESR or CRP, which can be useful in discriminating inflammatory from noninflammatory disorders. Both are inexpensive and easily obtained and may be elevated with infection, inflammation, autoimmune disorders, neoplasia, pregnancy, renal insufficiency, and advanced age.
Serum uric acid determinations are useful only when gout has been diagnosed and therapy contemplated. Uric acid, the end product of purine metabolism, is primarily excreted in the urine. Serum values range from 238-516 μmol/L (4.0-8.6 mg/dL) in men; the lower values [178-351 μmol/L (3.0-5.9 mg/dL)] seen in women are caused by the uricosuric effects of estrogen. Urinary uric acid levels are normally <750 mg per 24 h. Although hyperuricemia [especially levels >535 μmol/L (9 mg/dL)] is associated with an increased incidence of gout and nephrolithiasis, levels do not correlate with the severity of disease. Uric acid levels (and the risk of gout) may be increased by inborn errors of metabolism (Lesch-Nyhan syndrome), disease states (renal insufficiency, myeloproliferative disease, psoriasis), or drugs (alcohol, cytotoxic therapy, thiazides).Although nearly all patients with gout will demonstrate hyperuricemia at some time during their illness, up to 40% of patients with an acute gouty attack will have normal serum uric acid levels. Monitoring serum uric acid may be useful in assessing the response to hypouricemic therapy or chemotherapy.
Serologic tests for rheumatoid factor, cyclic citrulli-nated peptide (CCP) antibodies, ANAs, complement levels, Lyme and antineutrophil cytoplasmic antibodies (ANCA), or antistreptolysin O (ASO) titer should be carried out only when there is clinical evidence to suggest an associated diagnosis, as these have poor predictive value when used for screening, especially when the pretest probability is low. Although 4-5% of a healthy population will have positive tests for rheumatoid factor and ANAs, only 1% and <0.4% of the population will have RA or SLE, respectively. IgM rheumatoid factor (autoantibodies against the Fc portion of IgG) is found in 80% of patients with RA and may also be seen in low titers in patients with chronic infections (tuberculosis, leprosy); other autoimmune diseases (SLE, Sjögren’s syndrome); and chronic pulmonary, hepatic, or renal diseases. When considering RA, anti-CCP antibodies are comparably sensitive but more specific than rheumatoid factor. In RA, the presence of anti-CCP and rheumatoid factor antibodies may indicate a greater risk for more severe, erosive polyarthritis. ANAs are found in nearly all patients with SLE and may also be seen in patients with other autoimmune diseases (polymyositis, scleroderma, antiphospholipid syndrome), drug-induced lupus (resulting from hydralazine, procainamide, quinidine, tetracyclines, tumor necrosis factor inhibitors), chronic liver or renal disorders, and advanced age. Positive ANAs are found in 5% of adults and in up to 14% of elderly or chronically ill individuals. The ANA test is very sensitive but poorly specific for lupus, as <5% of all positive results will be caused by lupus alone. The interpretation of a positive ANA test may depend on the magnitude of the titer and the pattern observed by immunofluorescence microscopy (Table 17-4). Diffuse and speckled patterns are least specific, whereas a peripheral, or rim, pattern [related to autoantibodies against double-stranded (native) DNA] is highly specific and suggestive of lupus. Centromeric patterns are seen in patients with limited scleroderma (CREST syndrome) or primary biliary sclerosis, and nucleolar patterns may be seen in patients with diffuse systemic sclerosis or inflammatory myositis.
TABLE 17-4
|
ANTINUCLEAR ANTIBODY (ANA) PATTERNS AND CLINICAL ASSOCIATIONS |
||
|
ANA PATTERN |
ANTIGEN IDENTIFIED |
CLINICAL CORRELATE |
|
Diffuse |
Deoxyribonucleo- protein |
Nonspecific |
|
Histones |
Drug-induced lupus, lupus |
|
|
Peripheral (rim) |
ds-DNA |
50% of SLE (specific) |
|
Speckled |
U1-RNP |
>90% of MCTD |
|
Sm |
30% of SLE (specific) |
|
|
Ro (SS-A) |
Sjögren’s 60%, SCLE, neonatal lupus, ANA(-) lupus |
|
|
La (SS-B) |
50% of Sjögren’s, 15% lupus |
|
|
Scl-70 |
40% of diffuse scleroderma |
|
|
PM-1 |
Polymyositis (PM), dermatomyositis |
|
|
Jo-1 |
PM w/ pneumonitis + arthritis |
|
|
Nucleolar |
RNA polymerase I, others |
40% of PSS |
|
Centromere |
Kinetochore |
75% CREST (limited scleroderma) |
Note: SLE, systemic lupus erythematosus; MCTD, mixed connective tissue disease; SCLE, subacute cutaneous lupus erythematosus; PSS, progressive systemic sclerosis; CREST, calcinosis, Raynaud phenomenon, esophageal involvement; sclerodactyly; and felangiectasia.
Aspiration and analysis of synovial fluid are always indicated in acute monarthritis or when an infectious or crystal-induced arthropathy is suspected. Synovial fluid may distinguish between noninflammatory and inflammatory processes by analysis of the appearance, viscosity, and cell count. Tests for synovial fluid glucose, protein, lactate dehydrogenase, lactic acid, or autoantibodies are not recommended as they have no diagnostic value. Normal synovial fluid is clear or a pale straw color and is viscous, primarily because of the high levels of hyaluronate. Noninflammatory synovial fluid is clear, viscous, and amber colored, with a white blood cell count of <2000^L and a predominance of mononuclear cells. The viscosity of synovial fluid is assessed by expressing fluid from the syringe one drop at a time. Normally, there is a stringing effect, with a long tail behind each synovial drop. Effusions caused by OA or trauma will have normal viscosity. Inflammatory fluid is turbid and yellow, with an increased white cell count (2000-50,000^L) and a polymorphonuclear leukocyte predominance. Inflammatory fluid has reduced viscosity, diminished hyaluronate, and little or no tail following each drop of synovial fluid. Such effusions are found in RA, gout, and other inflammatory arthritides. Septic fluid is opaque and purulent, with a WBC count usually >50,000^L, a predominance of polymorphonuclear leukocytes (>75%), and low viscosity. Such effusions are typical of septic arthritis but may occur with RA or gout. In addition, hemorrhagic synovial fluid may be seen with trauma, hemarthrosis, or neuropathic arthritis. An algorithm for synovial fluid aspiration and analysis is shown in Fig. 17-6. Synovial fluid should be analyzed immediately for appearance, viscosity, and cell count. Monosodium urate crystals (observed in gout) are seen by polarized microscopy and are long, needle-shaped, negatively birefringent, and usually intracellular. In chondrocalcinosis and pseudogout, calcium pyrophosphate dihydrate crystals are usually short, rhomboidshaped, and positively birefringent. Whenever infection is suspected, synovial fluid should be Gram stained and cultured appropriately. If gonococcal arthritis is suspected, immediate plating of the fluid on appropriate culture medium is indicated. Synovial fluid from patients with chronic monarthritis should also be cultured for M. tuberculosis and fungi. Last, it should be noted that crystal-induced and septic arthritis occasionally occur together in the same joint.
FIGURE 17-6
Algorithmic approach to the use and interpretation of synovial fluid aspiration and analysis.
Diagnostic Imaging in Joint Diseases
Conventional radiography has been a valuable tool in the diagnosis and staging of articular disorders. Plain x-rays are most appropriate when there is a history of trauma, suspected chronic infection, progressive disability, or monarticular involvement; when therapeutic alterations are considered; or when a baseline assessment is desired for what appears to be a chronic process. However, in acute inflammatory arthritis, early radiography is rarely helpful in establishing a diagnosis and may only reveal soft-tissue swelling or juxtaarticular demineralization. As the disease progresses, calcification (of soft tissues, cartilage, or bone),joint space narrowing, erosions,bony ankylosis, new bone formation (sclerosis, osteophytes, or periostitis), or subchondral cysts may develop and suggest specific clinical entities. Consultation with a radiologist will help define the optimal imaging modality, technique, or positioning and prevent the need for further studies.
Additional imaging techniques may possess greater diagnostic sensitivity and facilitate early diagnosis in a limited number of articular disorders, and in selected circumstances and are indicated when conventional radiography is inadequate or nondiagnostic (Table 17-5). Ultrasonography is useful in the detection of soft-tissue abnormalities that cannot be fully appreciated by clinical examination. Although inexpensive, it is seldom the preferred method of evaluation. The foremost application of ultrasound is in the diagnosis of synovial (Baker’s) cysts, although rotator cuff tears and various tendon injuries may be evaluated with ultrasound by an experienced operator. Radionuclide scintigraphy provides useful information regarding the metabolic status of bone and, along with radiography, is well suited for total-body assessment of the extent and distribution of skeletal involvement. Radionuclide imaging is a very sensitive, but poorly specific, means of detecting inflammatory or metabolic alterations in bone or periarticular soft-tissue structures. The limited tissue contrast resolution of scintigraphy may obscure the distinction between a bony or periarticular process and may necessitate the additional use of MRI.
TABLE 17-5
|
DIAGNOSTIC IMAGING TECHNIQUES FOR MUSCULOSKELETAL DISORDERS |
|||
|
METHOD |
IMAGING TIME, h |
COSTa |
CURRENT INDICATIONS |
|
Ultrasoundh |
<1 |
+ |
Synovial cysts Rotator cuff tears Tendon injury |
|
Radionuclide scintigraphy |
|||
|
99mTc |
1-4 |
++ |
Metastatic bone survey Evaluation of Paget’s disease Acute and chronic osteomyelitis |
|
111In-WBC |
24 |
+++ |
Acute infection Prosthetic infection Acute osteomyelitis |
|
67Ga |
24-48 |
++++ |
Acute and chronic infection Acute osteomyelitis |
|
Computed tomography |
<1 |
+++ |
Herniated intervertebral disk Sacroiliitis Spinal stenosis Spinal trauma Osteoid osteoma Stress fracture |
|
Magnetic resonance |
1/2-2 |
+++++ |
Avascular necrosis |
|
imaging |
Osteomyelitis Intraarticular derangement and soft-tissue injury Derangements of axial skeleton and spinal cord Herniated intervertebral disk Pigmented villonodular synovitis Inflammatory and metabolic muscle pathology |
||
aRelative cost for imaging study.
hResults depend on operator.
Scintigraphy, using 99mTc, 67Ga, or 111In-labeled WBCs, has been applied to a variety of articular disorders with variable success (Table 17-5). Although [99mTc] pertechnate or diphosphate scintigraphy (Fig. 17-7) may be useful in identifying osseous infection, neoplasia, inflammation, increased blood flow, bone remodeling, heterotopic bone formation, or avascular necrosis, MRI is preferred in most instances. The poor specificity of 99mTc scanning has largely limited its use to surveys for bone metastases and Paget’s disease of bone. Gallium scanning utilizes 67Ga, which binds serum and cellular transferrin and lactoferrin, and is preferentially taken up by neutrophils, macrophages, bacteria, and tumor tissue (e.g., lymphoma).As such, it is primarily used in the identification of occult infection or malignancy. Scanning with 111In-labeled WBCs has been used to detect osteomyelitis and infectious and inflammatory arthritis. Nevertheless, the use of 111In-labeled WBC or 67Ga scanning has largely been replaced by MRI, except when there is a suspicion of prosthetic joint infections.
FIGURE 17-7
[99mTc]diphosphonate scintigraphy of the feet of a 33-year-old black male with reactive arthritis, manifested by sacroili-itis, urethritis, uveitis, asymmetric oligoarthritis, and enthesitis. This bone scan demonstrates increased uptake indicative of enthesitis involving the insertions of the left Achilles tendon, plantar aponeurosis, and right tibialis posterior tendon as well as arthritis of the right first interphalangeal joint.
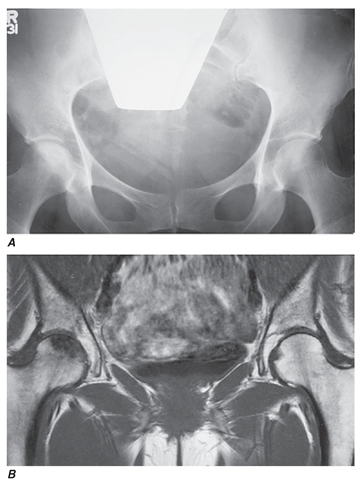
FIGURE 17-8
Superior sensitivity of MRI in the diagnosis of osteonecrosis of the femoral head. A 45-year-old woman receiving high-dose glucocorticoids developed right hip pain. Conventional x-rays (A) demonstrated only mild sclerosis of the right femoral head. T1-weighted MRI (B) demonstrated low-density signal in the right femoral head, diagnostic of osteonecrosis.
CT provides detailed visualization of the axial skeleton. Articulations previously considered difficult to visualize by radiography (e.g., zygapophyseal, sacroiliac, sternoclavicular, hip joints) can be effectively evaluated using CT. CT has been demonstrated to be useful in the diagnosis of low back pain syndromes (e.g., spinal stenosis vs. herniated disc), sacroiliitis, osteoid osteoma, and stress fractures. Helical or spiral CT (with or without contrast angiography) is a novel technique that is rapid, cost-effective, and sensitive in diagnosing pulmonary embolism or obscure fractures, often in the setting of initially equivocal findings. High-resolution CT can be advocated in the evaluation of suspected or established infiltrative lung disease (e.g., scleroderma or rheumatoid lung). The recent use of hybrid [positron emission tomography (PET)/CT or single photon emission CT (SPECT/CT)] scans in metastatic evaluations have incorporated CT to provide better anatomic localization of scintigraphic abnormalities.
MRI has significantly advanced the ability to image musculoskeletal structures. MRI has the advantages of providing multiplanar images with fine anatomic detail and contrast resolution (Fig. 17-8) that allows for the superior ability to visualize bone marrow and soft-tissue periarticular structures. Although more costly with a longer procedural time than CT, the MRI has become the preferred technique when evaluating complex musculoskeletal disorders.
MRI can image fascia, vessels, nerve, muscle, cartilage, ligaments, tendons, pannus, synovial effusions, and bone marrow. Visualization of particular structures can be enhanced by altering the pulse sequence to produce either T1- or T2-weighted spin echo, gradient echo, or inversion recovery [including short tau inversion recovery (STIR)] images. Because of its sensitivity to changes in marrow fat, MRI is a sensitive but nonspecific means of detecting osteonecrosis, osteomyelitis, and marrow inflammation indicating overlying synovitis or osteitis (Fig. 17-8). Because of its enhanced soft-tissue resolution, MRI is more sensitive than arthrography or CT in the diagnosis of soft-tissue injuries (e.g., meniscal and rotator cuff tears); intraarticular derangements; marrow abnormalities (osteonecrosis, myeloma); and spinal cord or nerve root damage or synovitis.

![[99mTc]diphosphonate scintigraphy of the feet of a 33-year-old black male with reactive arthritis, manifested by sacroili-itis, urethritis, uveitis, asymmetric oligoarthritis, and enthesitis. This bone scan demonstrates increased uptake indicative of enthesitis involving the insertions of the left Achilles tendon, plantar aponeurosis, and right tibialis posterior tendon as well as arthritis of the right first interphalangeal joint. [99mTc]diphosphonate scintigraphy of the feet of a 33-year-old black male with reactive arthritis, manifested by sacroili-itis, urethritis, uveitis, asymmetric oligoarthritis, and enthesitis. This bone scan demonstrates increased uptake indicative of enthesitis involving the insertions of the left Achilles tendon, plantar aponeurosis, and right tibialis posterior tendon as well as arthritis of the right first interphalangeal joint.](http://what-when-how.com/wp-content/uploads/2012/05/tmpa83a67_thumb22_thumb.png)