1. Introduction
The orbitrap mass analyzer is rightfully considered to be one of the newest analyzers. Its roots, however, can be traced back to 1923, when the principle of orbital trapping was proposed by Kingdon (1923). Experiments over the next half a century have shown that charged particles could be indeed trapped in electrostatic fields but they offered no hint of how this could be used for mass analysis. Simultaneously, advances in charged particle optics led to the development of a multitude of electrostatic fields (Korsunskii and Basakutsa, 1958). It was a quadro-logarithmic field used for orbital trapping of laser-produced ions that enabled Knight to perform crude mass analysis by applying axial resonant excitation to trapped ions (Knight, 1981). At the same time, this attempt demonstrated that the quest for a new mass analyzer would require a great deal of improvement in all key areas, most notably a more accurate definition of the quadro-logarithmic field, an ability to inject ions from an external ion source, and an improvement in ion detection.
These issues have been successfully addressed in the seminal work of Makarov (1999, 2000). A number of very significant technological advances were implemented, thus making the orbitrap analyzer usable in practical settings, namely, the development of pulsed injection from an external ion storage device (Hardman and Makarov, 2003; Hu et al., 2005; Makarov et al., 2006). Unique to the history of mass spectrometry, both proof of principle and product development have been carried out entirely within industry: from the first public announcement in 1999 till the entry into mainstream mass spectrometry in 2005.
The term orbitrap was first coined to define a mass analyzer wherein ions combine rotation around an electrode system with harmonic oscillations along the axis of rotation at a frequency characteristic of their m/q value (Makarov, 2000). The image current from coherently oscillating ions is detected on receiver plates as a time-domain signal. Fourier transform of this signal then gives us a mass spectrum. Therefore, the orbitrap analyzer extends the family of Fourier transform mass analyzers, which, until recently, contained only one widely used analyzer: Fourier transform ion cyclotron resonance (FTICR).
From the point of view of an ion optics design, the orbitrap analyzer belongs in the class of closed electrostatic traps. In theory, the ions could remain confined within such traps for an indefinite period of time. Other examples of closed electrostatic traps include ion storage rings, linear electrostatic rings, multiple-reflection ion mirrors, and so on. The harmonic nature of oscillations enables the orbitrap mass analyzer to provide the highest frequency and the highest quality of focusing in this group. This, in turn, gives the orbitrap analyzer its superior performance with respect to the dynamic range, mass accuracy, and resolving power that it is capable of achieving.
2. Fundamental principles of operation
2.1. Geometry of the analyzer
The geometry of the orbitrap mass analyzer is shown in Figure 1. The trap consists of an outer barrel-like electrode and a central spindlelike electrode along the axis. These electrodes are shaped in such a manner so as to produce the quadro-logarithmic potential distribution:
where r, z are cylindrical coordinates (z =0 being the plane of the symmetry of the field), C is a constant, k is field curvature, Rm is the characteristic radius. In this trap, stable trajectories combine rotation around the central electrode with oscillations along the axis, resulting in an intricate spiral (Makarov, 2000).
2.2. Motion of trapped ions
There are three characteristic frequencies of ion motion:
• frequency of rotation around the central electrode
• frequency of radial oscillations (between maximum and minimum radii)
• frequency of axial oscillations (along z-axis).
Figure 1 Diagram of the orbitrap mass analyzer showing a spiral trajectory of an ion
Only the last one is completely independent of initial velocities and coordinates of the ions. Therefore, only this frequency could be used for determinations of mass-to-charge ratios m/q:
wherein constant k comes from equation (1) and changes in proportion to the voltage between the central and the outer electrodes.
2.3. Ion detection
Axial oscillation frequencies can be directly detected by measuring the image current on the outer orbitrap electrodes as shown in Figure 1. A broadband detection is followed by a fast Fourier transformation (FFT) to convert the recorded time-domain signal into a mass-to-charge spectrum (Marshall and Verdun, 1990). The image current is amplified and processed exactly in the same way as for FTICR, resulting in a similar sensitivity and signal-to-noise ratios. However, there is a minor but important distinction: the square-root dependence originating from the electrostatic nature of the field causes a much slower drop in resolving power observed for ions of increased m/q. As a result, the orbitrap analyzer may outperform FTICR in this respect for masses above a particular m/q (typically, above 1-2000 m/z).
There could be an alternative way of detecting ions that follows the original proposal of Knight (1981): to excite ions axially using a voltage at a resonant frequency and to scan the mass range by sweeping this frequency. However, this approach would offer no advantage over conventional traps while at the same time lacking the ability to carry out MSn experiments. Therefore, detection of the image current remains the major mode of operation for the orbitrap mass spectrometers.
2.4. Formation of coherent ion packets
The most important prerequisite for detection of the image current is the ability to concentrate all ions of the same m/q. The relevant dimensions of the ion cloud must be smaller than the amplitude of oscillations we wish to detect. This could be achieved in one of two ways:
• Broadband excitation of ions from the equatorial plane. Traditionally, this approach is used in FTICR and is compatible with well-known types of external ratio frequency (RF) storage devices. But such an approach demands substantial complexity of an ion introduction apparatus (Makarov, 1999).
• Excitation by off-axis injection of pulsed ion packets (“excitation by injection”). This approach minimizes perturbations of the quadro-logarithmic field but requires a very fast ejection of large ion population from an ion source or an external RF storage device.
Ultimately, the second approach has proved to be more practical and robust. It is performed according to the following sequence:
1. Ions are trapped in a gas-filled RF-only set of rods, preferably a linear ion trap. In principle, this also allows various manipulations of the ions, including isolation, fragmentation, MSn, and so on.
2. Pulsed voltages are applied to the end electrodes (Hardman and Makarov, 2003; Hu et al., 2005) or across the RF electrodes (Makarov et al., 2006) so that the ions find themselves in a strong extraction field. The probability of collisions and collision-induced dissociation during the ion extraction is minimized by storing ions near the exit orifice. Additional lenses are used for the final spatial focusing of the ion beam into the entrance of the orbitrap analyzer, as well as to facilitate differential pumping to achieve the very high vacuum necessary for effective mass measurement.
3. Ions of individual mass-to-charge ratios arrive at the entrance of the orbitrap analyzer as a tight packet with dimensions considerably smaller than the amplitude of their axial oscillations. When ion packets are injected into the orbitrap analyzer off-axis (Figure 1), they start coherent axial oscillations without the need for any additional excitation.
4. Upon entering the orbitrap analyzer, the ion packets are “squeezed” by increasing the electric field to move the ions toward the equator and the central electrode. This increase in electric field is created by ramping up the voltage on the central electrode.
5. Because of the strong dependence of rotational frequencies on ion energies, angles, and initial positions, each ion packet soon spreads over the angular coordinate, forming a thin rotating ring. This has important ramifications: more ions can be present in the orbitrap mass analyzer before the space charge effects start impacting the mass resolution and accuracy of the measurement.
Following the injection, the voltages on both the central electrode and deflector are stabilized so that no mass drift can take place during the next stage (ion detection).
2.5. Decay of coherent ion packets
As mentioned earlier, under ideal conditions, the ions could remain in an electrostatic analyzer indefinitely. Unfortunately, the collisions with residual gas in the orbitrap cause ions to scatter and limit the time a transient can be detected for a period of a few seconds. A decay of the transient in the orbitrap mass analyzer and consequent limitation of resolving power is further caused by a loss of coherence due to miniscule imperfections of the orbitrap electrode manufacturing, and due to space charge repulsion. The transient could be further extended by improving the accuracy of electrodes and the vacuum.
The space charge repulsion is greatly reduced because of the shielding action of the central electrode that screens ions on one side of the ion ring from influencing ions on the other side. However, a due diligence in the design of the orbitrap electrodes is needed to avoid more complex nonlinear effects caused by the interaction between nonlinear field perturbations and space charge.
2.6. Main analytical parameters of the orbitrap mass analyzer
Similar to other mass analyzers (e.g., a quadrupole), analytical parameters are determined to a large extent by the present status of manufacturing technology and electronics. The current level of machining precision enables the resolving power of the orbitrap mass analyzer to reach a value of several hundred thousands. Internal and thermal noise of electronic components impacts the sensitivity of detection of the image current in the orbitrap, making a couple of dozen ions its limit of detection. The mass error using external mass calibration is a few parts per million and remains stable over a 24-hour period. The accuracy is limited principally by the noise of electronic components. The repetition rate of a few hertz reflects the present speed at which we are able to apply a high voltage to the central electrode. While this combination of analytical parameters remains inferior to that of FTICR mass analyzers, it appears to be attractive enough given the absence of a superconducting magnet and of lower m/q ion losses suffered during transfer from an external ion storage device.
In comparison with other accurate-mass analyzers (e.g., time-of-flight analyzers), the orbitrap system offers much higher dynamic range over which accurate masses can be determined (extent of mass accuracy).
2.7. Fragmentation in the orbitrap mass analyzer
The ions trapped in the orbitrap analyzer have energies in the range of kiloelectron volts. A high-energy fragmentation caused by collision with residual gas happens automatically, and its extent could be regulated by gas pressure. Pulsed lasers could be another way to induce fragmentation of ions in the orbitrap. Unfortunately, when an ion decays under the dynamic trapping, its fragments will have the same velocity. As their energy is proportional to their individual mass-to-charge ratios, the trajectories become highly elliptical. Therefore, low-mass fragments (with m/q typically below 30-50% of that of the precursor ion) will fall onto the central electrode, while lower-charge state fragments (with m/q typically above 50% of the precursor ion) will hit the outer electrodes. This property seriously limits the utility of the analyzer for MSn, especially taking into consideration the absence of collisional cooling, increased cycle time, inferior resolving power and sensitivity, cost, and complexity of such an apparatus.
2.8. The orbitrap analyzer used as an accurate-mass detector in hybrid mass spectrometers
The challenging nature of the technical issues related to performing MS/MS in the orbitrap mass analyzer was the main reason behind the concept of using it as an accurate-mass detector for another mass analyzer: linking two mass spectrometers into one hybrid instrument. If we also insert an ion storage device between the first mass analyzer and the orbitrap, the analyzers are effectively decoupled from each other and any mass analyzer could be used as the first stage.
In the first commercial orbitrap-based instrument, a linear ion trap with radial ejection (Schwartz 2005) was chosen as a “partner” for the orbitrap mass analyzer because of its very high sensitivity, superb control of the ion population, short cycle time, and MSn capability. Depending on the requirements for the analysis, the two analyzers can be used independently or in concert. It is worth pointing out that the MS/MS spectra generated in the linear ion trap and the orbitrap mass analyzer are very similar, with the only major difference being the resolution and mass accuracy of the observed peaks.
The ion storage device linking the linear ion trap to the orbitrap analyzer is called the C-trap. The C-trap has a high space charge capacity (Makarov etal., 2006), and enables several intriguing modes of operation:
• Ions can be fragmented by injecting them into the C-trap at higher energies to yield fragmentation patterns similar to those in triple-quadrupole mass spectrometers.
• The C-trap supports multiple fills. An injection of a fixed number of ions of a known compound can be followed by injection of analyte ions. Both sets of ions are then injected simultaneously into the orbitrap. This allows for a robust internal calibration of each spectrum.
• Multiple injections of ions fragmented or selected at different conditions could be stored together and acquired in a single orbitrap spectrum.
• Additional fragmentation methods could be used within the ion storage device, for example, IRMPD (infrared multiphoton dissociation), ion-molecule reactions, and ETD (electron transfer dissociation).
The next section describes selected analytical applications of the hybrid LTQ orbitrap mass spectrometer.
3. Analytical applications of the orbitrap mass analyzer
The key attributes of the orbitrap analyzer, namely, ruggedness, high mass accuracy, and excellent resolving power, make it suitable for both proteomics and small-molecule analyses. Details regarding recently published proteomics applications can be found in Scigelova and Makarov (2006), and a short overview is presented here.
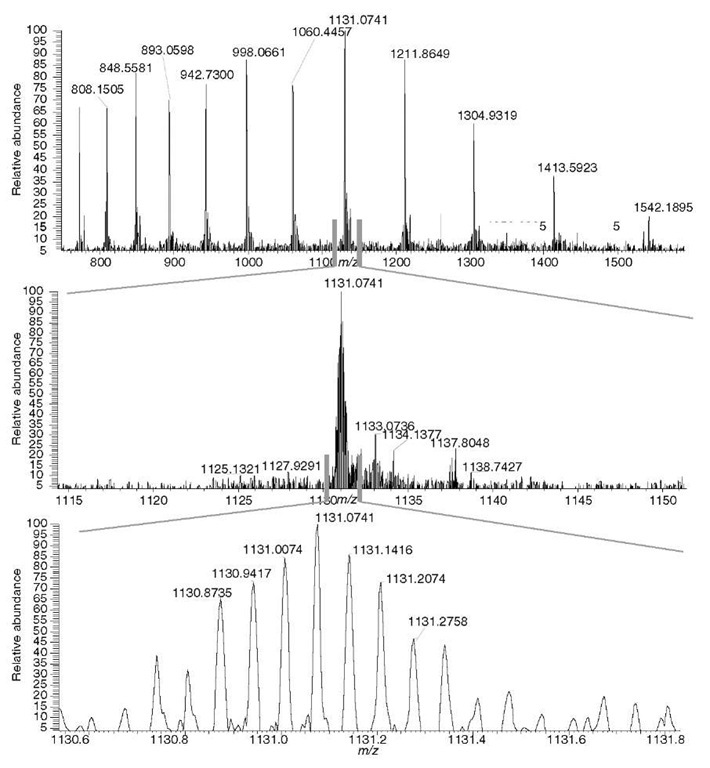
The main benefit to the user is that a very accurately measured mass of a peptide allows for a considerable reduction of false positive identifications, thus resulting in a highly confident identification. The high resolving power helps deal effectively with extremely complex peptide mixtures. Figure 2 illustrates this point; the mass of the two coeluting isobaric peptides can be accurately determined only if peaks are sufficiently resolved.
On the other hand, the fragmentation (MS/MS) spectra of peptides for database searches might not be necessarily required with such high precision. When MS/MS spectra is recorded with the linear ion trap detector, three spectra per second can be comfortably obtained. Both mass analyzers can indeed work in parallel: while a high-resolution/mass accuracy spectrum of the precursor is being acquired in the orbitrap, the fast linear ion trap carries out fragmentation and detection of MS/MS (or higher-order MSn) spectra of selected peptides. A true parallel operation is achieved by allowing for a short preview of ions that are being measured in the orbitrap analyzer. This preview defines the parent ions that the linear ion trap will fragment. Within one second, one orbitrap spectrum acquired at resolving power 60 000 together with three linear ion trap fragmentation spectra can be obtained (Makarov et al., 2006).
Figure 2 Analyzing isobaric peptides. The two peptides need to be sufficiently resolved (resolving power 60 000 was used in the experiment) to allow for an accurate-mass assignment
Analysis of posttranslational modifications is an area of proteomics that greatly benefits from accurate-mass measurement as well as multiple levels of fragmentation (Olsen et al., 2006). An accurate measurement of the neutral loss of phosphate from the precursor ion in the MS/MS spectrum allows for a selective targeting of phosphopeptides in complex mixtures, while the site of phosphorylation is confidently identified in the following MS3 spectrum.
De novo sequencing of peptides is arguably the biggest challenge in proteomics. The problem is compounded by a “combinatorial explosion” caused when considering multiple modifications. Mass accuracy and high resolution considerably improve the results of de novo interpretation of peptide MS/MS spectra using computer algorithms (Figure 3).
The orbitrap mass analyzer offers sufficiently high resolving power to encourage attempts at analyzing medium-sized proteins (10-25 kDa, Figure 4). This approach, called a top-down analysis, enables a detailed characterization of the protein, including the determination of posttranslational modifications.
In the areas related to small molecule analysis, the ability to conduct biotrans-formation profiling via tandem mass spectrometry coupled with accurate-mass measurement, all in a single experiment, is clearly one of the most attractive features of the orbitrap mass analyzer. The tight mass tolerance reduces or eliminates background chemical noise. Thus, suspected metabolites can be confirmed or refuted using (1) the predictive chemical formula and corresponding mass error of the analysis, (2) ring-plus double bond equivalent rule, and (3) accurate-mass measurement of product ion spectra of suspected metabolites (Peterman et al., 2006).
Figure 3 De novo sequence assignment of a snail peptide. Note the presence of three modified amino acid residues. It is worth pointing out that the orbitrap mass analyzer confidently differentiates between oxidized methionine and phenylalanine candidates (mass difference 0.033 u).
In the context of whole biological systems, the study of metabolic networks has so far been hindered by the lack of techniques that identify metabolites and their biochemical relationship. The orbitrap mass analyzer is proving to be very useful in this field (Breitling et al., 2006).
In some areas of research, the use of a high-resolution/accurate-mass analyzer with MSn capabilities is poised to cause a major shift in experimental approaches and strategies. For instance, the study of lipid mixtures traditionally using parent and neutral ion scanning on triple-quadrupole mass spectrometers is now able to adopt a “profiling” approach relying on high resolution/accurate mass (Ejsing et al., 2006). This approach has the potential to revolutionize the study of lipids, making it a high-throughput global approach akin to biomarker discovery in the areas of proteomics and metabolomics.
Yet another interesting application of the orbitrap mass spectrometer to small molecule analysis is in the area of drugs of abuse. The orbitrap analyzer allows for a thorough characterization of novel compounds that have a high potential for misuse in sports (Thevis et al., 2006a, 2006b). The MSn capability of the hybrid linear ion trap-orbitrap mass spectrometer is used to determine fragmentation pathways while a routine analysis is then conducted on a triple-quadrupole mass spectrometer.
Figure 4 Intact myoglobin is measured in the orbitrap mass analyzer at resolving power 100 000 in an infusion experiment.
In conclusion, it is clear that the orbitrap mass analyzer is fast becoming a unique and powerful addition to the scientific toolbox for probing biological systems and increasing selectivity and confidence of routine analyses. The above shortlist of applications is bound to expand considerably in the near future as the orbitrap systems are becoming more widespread and penetrate into other areas of research. Undoubtedly, we will see major breakthroughs in the design and development of the orbitrap analyzer technology.