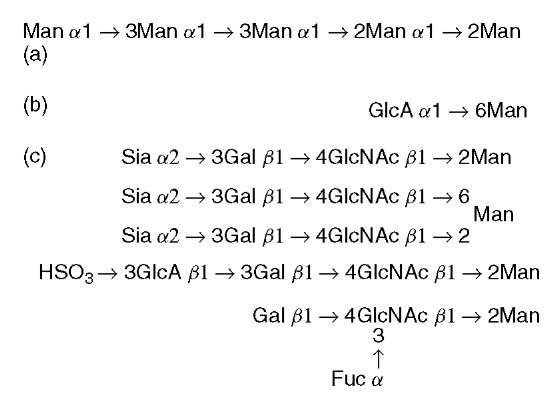1. Introduction
Over 60% of the proteins produced by the human body are thought to contain sugar chains. Recent data have revealed the importance of sugar chains as biosignals for multicellular organisms, including cell-cell communication, intracellular signaling, protein folding, and targeting of proteins within cells. There is growing evidence that sugar chains have a variety of roles in cellular differentiation and development, as well as in disease processes.
The major sugar chains of glycoproteins can be classified into two groups according to their sugar-peptide linkage regions. Those that are linked to asparagine (Asn) residues of proteins are termed N -glycans (see Article 64, Structure/function of N -glycans, Volume 6), while those that are linked to serine (Ser) or threonine (Thr) residues are called O -glycans (see Article 65, Structure/function of O -glycans, Volume 6). In N-glycans, the reducing terminal N-acetylglucosamine (GlcNAc) is linked to the amide group of Asn via an aspartylglycosylamine linkage. In O -glycans, the reducing terminal N-acetylgalactosamine (GalNAc) is attached to the hydroxyl group of Ser and Thr residues. In addition to the abundant O-GalNAc forms, several unique types of protein O -glycosylation have been found, such as O -linked fucose, glucose, GlcNAc (see Article 72, O-linked N -acetylglucosamine (O-GlcNAc), Volume 6), and mannose, which have been shown to mediate diverse physiological functions. For example, O-mannosylation has recently been shown to be important in muscle and brain development.
2. Structure of O -mannosyl glycan
O -Mannosylation is known as a yeast-type modification, and O -mannosylated glycoproteins are abundant in the yeast cell wall (Strahl-Bolsinger et al., 1999). In unicellular eukaryotic organisms, all O -mannosyl glycan structures elucidated so far are neutral linear glycans consisting of 1 to 7 mannose residues (Figure 1). O -Mannosylation of proteins has been shown to be vital in yeast, and its absence may affect cell wall structure and rigidity. Additionally, a deficiency in protein O-mannosylation in the fungal pathogen Candida albicans leads to defects in multiple cellular functions, including expression of virulence. In addition to fungi and yeast, clam worm has an O -mannosyl glycan (a glucuronyla1-6mannosyl disaccharide) in skin collagen (Spiro and Bhoyroo, 1980).
Figure 1 O-Mannosyl glycans found in (a) yeast, (b) clam worm, and (c) mammals. Man: mannose; GlcA: glucuronic acid; Sia: sialic acid; Gal: galactose; GlcNAc: N -acetylglucosamine; Fuc: fucose
Mammalian O -mannosylation is an unusual type of protein modification that was first identified in chondroitin sulfate proteoglycans of brain, and is present in a limited number of glycoproteins of brain, nerve, and skeletal muscle (Endo, 1999). In brief, one of the best known O-mannosyl-modified glycoproteins is a-dystroglycan, which is a central component of the dystrophin-glycoprotein complex isolated from skeletal muscle membranes. We previously found that the glycans of a-dystroglycan include O -mannosyl oligosaccharides and that a sialyl O -mannosyl glycan, Siaa2-3Galj 1-4GlcNAcj 1-2Man, is a laminin-binding ligand of a-dystroglycan (Chiba et al., 1997). Interestingly, we found the same O-mannosyl glycan in rabbit skeletal muscle a-dystroglycan. After our reports of the sialylated O -mannosyl glycan, an HNK-1 epitope (sulfoglucuronyl lactosamine) carrying O-mannosyl glycan (HSO3-3GlcAj 1-3Galj 1-4GlcNAcj 1-2Man) was detected in total brain glycopeptides. It is noteworthy that these oligosaccharides have not only 2-substituted mannose but also 2,6-disubstituted mannose. Further, dystro-glycan from sheep brain has a Galj 1-4(Fuca1-3)GlcNAcj 1-2Man structure and mouse J1/tenascin, which is involved in neuron-astrocyte adhesion, contains the O-mannosyl glycans. Therefore, it is likely that a series of O-mannosyl glycans, with heterogeneity of mannose-branching and peripheral structures, is present in mammals. Further studies are needed to clarify the distribution of such O-mannosyl glycans in various tissues.
3. Biosynthesis of O -mannosyl glycan
Identification and characterization of the enzymes involved in the biosynthesis of mammalian type O -mannosyl glycans will help to elucidate the function and regulation of expression of these glycans.
A key difference among yeast, clam worm, and mammalian-type O -mannosyl glycans is that those in mammals have the GlcNAc j1-2Man linkage (Figure 1).
This linkage is assumed to be catalyzed by a glycosyltransferase, UDP-GlcNAc: protein O-mannose j1,2-N-acetylglucosaminyltransferase (POMGnTl). POMGnTl catalyzes the transfer of GlcNAc from UDP-GlcNAc to O-mannosyl glycoproteins, and human POMGnTl gene was cloned (Yoshida et al., 2001). The nucleotide sequence indicated that human POMGnT1 is a 660 amino acid protein and is a type II membrane protein. This topology was similar to the topologies of other Golgi glycosyltransferases. As described already, mammalian O-mannosyl glycan has 2,6-disubstituted mannose. Very recently, a gene for this 6-branching enzyme (GnT-IX) has been cloned (Inamori et al., 2004).
In yeast, the family of protein O -mannosyltransferases catalyzes the transfer of a mannosyl residue from dolichyl phosphate mannose (Dol-P-Man) to Ser/Thr residues of certain proteins (Strahl-Bolsinger etal., 1999). However, attempts to detect protein O -mannosyltransferase activity and to characterize the enzyme(s) responsible for the biosynthesis of O -mannosyl glycans in vertebrates have not been successful. Two human homologs, POMT1 and POMT2, were found but their gene products did not show any protein O -mannosyltransferase activity (Jurado etal., 1999; Willer etal., 2002). POMT1 and POMT2 share almost identical hydropathy profiles that predict both to be integral membrane proteins with multiple transmembrane domains. Recently, we developed a new method to detect the enzymatic activity of protein O-mannosyltransferase in mammalian cells and tissues. Using this new method, we demonstrated that human POMT1 and POMT2 have protein O -mannosyltransferase activity, but only when they are coexpressed (Manya etal., 2004). This suggests that POMT1 and POMT2 form a hetero-complex to express enzymatic activity similar to the complex in yeast (Girrbach and Strahl, 2003). POMT1 and POMT2 are expressed in all human tissues, but POMT1 is highly expressed in fetal brain, testis, and skeletal muscle, and POMT2 is predominantly expressed in testis (Jurado et al., 1999; Willer et al., 2002). O-Mannosylation seems to be uncommon in mammals, and only a few O-mannosylated proteins have been identified. It will be of interest to determine the regulatory mechanisms for protein O -mannosylation in each tissue.
Enzymes for galactosylation, sialylation, fucosylation, glucuronylation, or sulfation of O -mannosyl glycans are not identified yet.
4. Defects of O -mannosylation and congenital muscular dystrophies
Muscular dystrophies are genetic diseases that cause progressive muscle weakness and wasting (Burton and Davies, 2002). Recent data suggest that aberrant O-mannosylation of a-dystroglycan is the primary cause of some forms of congenital muscular dystrophy and neuronal migration disorder.
Muscle-eye-brain disease (MEB: OMIM 253280) is an autosomal recessive disorder characterized by congenital muscular dystrophy, ocular abnormalities, and brain malformation (type II lissencephaly). MEB has been observed mainly in Finland. After we screened the entire coding region and the exon/intron flanking sequences of the POMGnTl gene for mutations in patients with MEB, we identified 13 independent disease-causing mutations in these patients (Taniguchi et al., 2003; Yoshida etal., 2001). We have not detected these 13 substitutions in any of the 300 normal chromosomes, indicating that they are pathogenic and that the POMGnTl gene is responsible for MEB. To confirm that the mutations observed in patients with MEB are responsible for the defects in the synthesis of O -mannosyl glycan, we expressed all of the mutant proteins and found that none of them had enzymatic activity (Manya et al., 2003; Yoshida et al., 2001). Together, these findings indicate that MEB is inherited as a loss-of-function of the POMGnTl gene. If POMGnT1 does not work, no peripheral structure can be formed on O-mannosyl glycans. Because these structures are involved in adhesive processes, a defect of O-mannosyl glycan may severely affect cell migration and cell adhesion. Additionally, a selective deficiency of a-dystroglycan in MEB patients was found. This finding suggests that a-dystroglycan is a potential target of POMGnT1 and that hypoglycosylation of a-dystroglycan may be a pathomechanism of MEB. MEB muscle and brain phenotypes can be explained by abnormal O -mannosylation.
Walker-Warburg syndrome (WWS: OMIM 236670) is another form of congenital muscular dystrophy that is characterized by severe brain malformation (type II lissencephaly) and eye involvement. Patients with WWS are severely affected from birth and usually die within their first year. WWS has a worldwide distribution. Recently, 20% of WWS patients (6 of 30 unrelated WWS cases) has been found to have mutations in POMT1 (Beltran-Valero de Bernabe et al., 2002), but none of the 30 cases studied had mutations in another homolog, POMT2. This suggests that other as yet unidentified genes are responsible for this syndrome.
In WWS patients, as in MEB patients, a highly glycosylated a-dystroglycan was selectively deficient in skeletal muscle. WWS and MEB are clinically similar disorders, but WWS is a more severe syndrome than MEB. The difference of severity between the two diseases may be explained as follows. If POMGnT1, which is responsible for the formation of the GlcNAc j 1-2Man linkage of O -mannosyl glycans, is nonfunctional, only O -mannose residues may be present on a-dystroglycan in MEB. On the other hand, POMT1 mutations cause complete loss of O-mannosyl glycans in WWS. It is possible that the attachment of a single mannose residue on a-dystroglycan in MEB is responsible for the difference in the clinical severity of WWS and MEB.
Interestingly, the rt mutation in Drosophila, which causes defects of myogenesis, was found to be due to a mutation in a homolog of POMT1 (Martin-Blanco and Garcia-Bellido, 1996; Willer etal., 2002). Although the rt gene product is not known to initiate the biosynthesis of O -mannosyl glycans, O -mannosylation is an evolutionarily conserved protein modification (Endo, 1999), and may be essential for muscle development in both vertebrates and invertebrates.
After reporting that MEB and WWS are caused by defects of O -mannosylation, some muscular dystrophies have been suggested to be caused by abnormal glycosy-lation of a-dystroglycan, for example, Fukuyama-type congenital muscular dystrophy (FCMD: OMIM 253800), congenital muscular dystrophy type 1 C (MDC1C: OMIM 606612), congenital muscular dystrophy type 1D (MDC1D), and the myodystrophy (myd) mouse. However, although highly glycosylated a-dystroglycan was found to be selectively deficient in the skeletal muscle of these patients and the gene products were thought to be putative glycosyltransferases, it is still unclear due to the defects of O-mannosylation. Identification of these defects may provide new clues to the glycopathomechanism of muscular dystrophy.
5. Conclusions
O -Mannosylation is an uncommon protein modification in mammals, but it is important in muscle and brain development. Since a few O -mannosylated proteins have been identified, further proteomic studies are needed to clarify the distribution of O -mannosyl glycans in various tissues and to examine their changes during development. Future studies may also reveal that presently uncharacterized forms of muscular dystrophy are caused by defects in other glycosyltransferases. A major challenge will be to integrate the forthcoming structural, cell biological, and genetic information to understand how a-dystroglycan O -mannosylation contributes to muscular dystrophy and neuronal migration disorder.