The main objective of a petroleum refinery is the production of fuels (e.g., gasoline, diesel). Straight-run distillates cannot be used directly as fuels since they possess high amounts of impurities and octane and cetane numbers that are not appropriate for gasoline and diesel engines. These straight-run distillates need treatment to make them suitable for fuel production, which is carried out in various refining processes, as illustrated in Figure 1.6. A brief description of the fundamentals of the various processes used for fuels production is presented in this section. More details on the most important refining processes are given in subsequent topics.
Figure 1.6. Typical process scheme of a petroleum refinery.
Catalytic Reforming
Catalytic reforming is used to convert low-octane straight-run naphtha into high-octane gasoline, called reformate, and to provide aromatics (BTX: benzene, toluene, and xylene) for petrochemical plants. The reformate has higher aromatic and cyclic hydrocarbon contents. The main reactions occurring in catalytic reforming are:
• Dehydrogenation of naphthenes to aromatics
• Isomerization of paraffins to branched-chain structures
• Isomerization of naphthenes
• Dehydrocyclization of paraffins and olefins to aromatics
• Hydrocracking of high – boiling hydrocarbons to low – molecular- weight paraffins (hydrocracking of paraffins is undesirable due to increased light ends made)
The objective of these reactions is to restructure and crack some of the molecules present in the feed to produce a product with hydrocarbons that have more complex molecular shapes, whose overall effect is the production of a reformate with a higher octane number than that of the feed. Apart from producing high-octane gasoline, catalytic reforming also produces very significant amounts of hydrogen gas as a by-product, which is released during catalyst reaction and is used in other processes within the refinery (e.g., catalytic hydrotreating and hydrocracking).
A typical catalytic reforming process includes the following steps (Figure 1.7 ):
• Mixing the feed (naphtha) with recycle hydrogen, heating, and passing through a series of catalytic reactors. The feed must be almost free of sulfur, since even in extremely low concentrations, it poisons the noble metal catalysts (platinum and rhenium) used in the catalytic reforming units.
• Since most of the reactions are highly endothermic, each reactor effluent is reheated before entering the following reactor.
• The effluent from the final reactor is separated into hydrogen-rich gas and reformate, and the hydrogen is recycled or purged for using in other processes. Hydrogen recycle reduces the formation of carbon.
• Reformate product is sent to gasoline blending.
Isomerization
Isomerization is an ideal choice to produce a gasoline blending component from light paraffins. The objective of isomerization is to convert low-octane n- paraffins to high-octane i- paraffins by using a chloride-promoted fixed – bed reactor. The main steps of a typical isomerization process are (Figure 1.8):
Figure 1.7. Typical process scheme of a catalytic reforming unit.
Figure 1.8. Typical process scheme of an isomerization unit.
• Drying the previously desulfurized feed and hydrogen in fixed beds of solid desiccant prior to mixing together
• Heating the mixed feed and passing it through a hydrogenation reactor to saturate olefins to paraffins and to saturate benzene
• Cooling the hydrogenation effluent and passing it through an isomeriza-tion reactor, where the isomerization reaction takes place in the catalyst bed
• Cooling the final effluent first by heat exchange with the incoming feed and then by water or air cooling
• Separating the cooled effluent into hydrogen and a liquid stream
• Sending the liquid stream to a reboiled stripper column, where a debu-tanized isomerate liquid leaves as the bottom product, and the butanes and lighter components leave at the top
• Partially condensing to the gas stream provide reflux to the column and a liquid product rich in butanes and propane (LPG)
• When it leaves the stripper condenser drum, sending the uncondensed overhead to the fuel gas.
• Sending the debutanized isomerate as a product for gasoline blending
As result of the isomerization reactions, highly branched, high-octane paraf-finic blending components are obtained, which by themselves can satisfy the strictest gasoline environmental requirements. However, production of this isomerate is low, and other streams for gasoline blending are still necessary. Isomerization of n -butane is also one source for the isobutane required in alkylation.
Alkylation
The objective of the alkylation process is to combine light olefins (primarily a mixture of propylene and butylene) with isobutane to form a high- octane gasoline (highly branched C5 – C12 i- paraffins), called alkylate. The major constituents of alkylate are isopentane and isooctane (2,2,4-trimethyl pentane), the latter possessing an octane number of 100. Among all refinery processes, alkylation is a very important process that enhances the yield of high-octane gasoline. The reaction occurs in the presence of a highly acidic liquid catalyst (HF: hydrofluoric acid or H2SO4: sulfuric acid). As a consequence of the environmental problems associated with the use of these liquid catalysts, solid acid catalysts have also been proposed, having as a major problem rapid deactiva-tion due to coke formation.
The main steps of a typical hydrofluoric alkylation unit are (Figure 1.9):
• Mixing the olefins coming from fluid catalytic cracking process with isobutane and feeding the mixture to the reactor where the alkylation reaction occurs. Prior to mixing, the olefin feed needs pretreatment to remove H2S and mercaptans.
Figure 1.9. Typical process scheme of an alkylation unit.
• Separation of the free HF from the hydrocarbons in an acid settler and recycling the acid back to the reactor.
• Regeneration of part of the HF to remove acid oils formed by feed contaminants or hydrocarbon polymerization.
• Sending the hydrocarbons from the acid settler to the de-isobutanizer, where propane and isobutane are separated from n- butane and alkylate.
• Fractionation of propane from isobutane. Isobutane in then recycled to the reactor.
• n-Butane and alkylate are defluorinated in a bed of solid adsorbent and fractionated as separate products. Propane and n-butane are nonreactive hydrocarbons.
The function of the acid catalyst is to protonate the olefin feed to produce reactive carbocations, which alkylate isobutane. Alkylation reaction is very fast with 100% olefin conversion. It is important to keep a high isobutene-to-olefin ratio to prevent side reactions, which can produce a lower-octane product. This is the reason that alkylation units have a high recycle of isobutane.
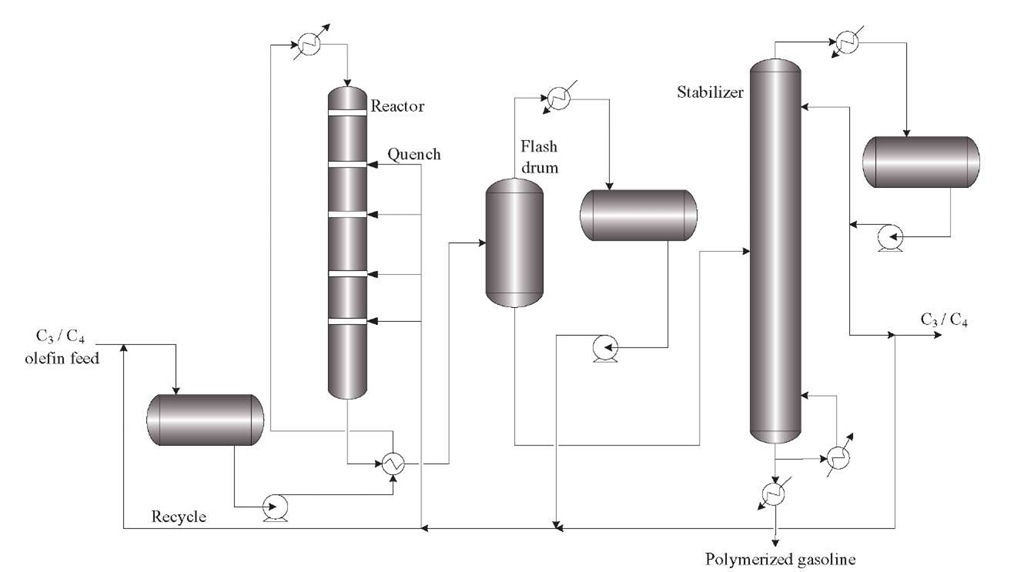
Polymerization
The objective of a polymerization unit is to combine or polymerize the light olefins propylene and butylene into molecules two or three times their original molecular weight. The feed to this process consists of light gaseous hydrocarbons (C3 and C4) produced by catalytic cracking, which are highly unsaturated. The polymer gasoline produced has octane numbers above 90. Although the amount of polymer gasoline is very small, it is an important part of a refinery since the polymerization process increases the yield of gasoline possible from gas oil. For example, the numbers of barrels of polymer gasoline per barrel of olefin feed is about half those of alkylate, but capital and operating costs are much lower in polymerization because it operates at low pressures compared with alkylation. The polymerization reaction consists of passing the C–C4 hydrocarbon stream with a high proportion of olefins through a reactor containing a phosphoric acid-supported catalyst, where the carbon-carbon bond formation occurs.
Polymerization comprises the following main steps (Figure 1.10):
• Contacting the feed with an amine solution to remove H2S and washing with caustic to remove mercaptans
• Scrubbing with water to remove any caustic or amines
• Drying by passing through a silica gel or molecular sieve bed
Figure 1.10. Typical process scheme of a polymerization unit.
• Adding a small amount of water to promote ionization of the acid before heating the olefin feed stream and passing over the catalyst bed
• Injecting a cold propane quench or by generating steam to control the reaction temperature since the polymerization reaction is highly exothermic
• Fractionating the product after leaving the reactor to separate the butane and lighter hydrocarbons from the polymer gasoline
Catalytic Hydro treating
Catalytic hydrotreating (HDT) is one of the most important processes in the petroleum refining industry. The HDT process is applied to treat a great variety of refinery streams, such as straight- run distillates, vacuum gas oils [fluid catalytic cracking (FCC) feed], atmospheric and vacuum residua, light cycle oil, FCC naphtha, and lube oils. The main differences in the hydrotreating processes of each feed are the operating conditions, type of catalyst, reactor configuration, and reaction system. Depending on the feed and the main objective of the treatment, the process can be called hydrodesulfurization (HDS), as in the case of the HDS of straight-run naphtha, which is used as reforming feed where sulfur is the main undesirable heteroatom. For straight-run gas oil, the process is called hydrotreating because, in addition to sulfur removal, aromatic saturation and nitrogen removal are also desired for diesel fuel production. A hydrodemetallization process is used for the removal of vanadium and nickel from heavy oils. When a change in the molecular weight of the feed is required, a hydrocracking process is used.
Sulfur is removed primarily to reduce the sulfur dioxide (SO2 ) emissions caused during fuel combustion. Removal of sulfur is also desired to have better feed for subsequent processes (e.g., catalytic reforming, fluid catalytic cracking). For naphtha HDS it is necessary to remove the total sulfur from the feed down to a few parts per million to prevent poisoning the noble metal catalysts in the catalytic reforming. For gas oil HDS, the production of ultralow-sulfur diesel (ULSD) requires the use of highly selective catalyst together with appropriate reaction conditions.
During hydrotreating a number of reactions are carried out: hydrogenolysis, by which C-S, C-N or C-C bonds are cleaved, and hydrogenation of unsatu-rated compounds. The reacting conditions of the HDT process vary with the type of feedstock; whereas light oils are easy to desulfurize, the desulfurization of heavy oils is much more difficult. The hydrotreating reactions take place in catalytic reactors at elevated temperatures and pressures, typically in the presence of a catalyst consisting of an alumina base impregnated with cobalt, nickel, and molybdenum. A typical hydrotreating unit involves the following steps (Figure 1.11):
• Mixing the liquid feed with a stream of hydrogen-rich recycle gas.
• Heating the resulting liquid-gas mixture to the desired reaction temperature.
Figure 1.11. Typical process scheme of a hydro treating unit.
• Feeding the mixture to the catalytic reactor, where the hydrotreating reactions take place.
• Cooling the reaction products and feeding them to a gas separator vessel.
• Sending most of the hydrogen-rich gas separated from this vessel through an amine contactor for removal of H2S.
• Recycling the H2S -free hydrogen-rich gas to the reactor.
• Sending the liquid from the gas separator vessel through a stripper distillation tower. The bottoms product from the stripper is the final desulfur-ized liquid product, while the overhead sour gas (i.e., hydrogen, methane, ethane, H-S, propane, butane, and some heavier components) is sent to the amine gas treating. Subsequently, the H2S removed and recovered is converted to elemental sulfur in a Claus process unit.
Fluid Catalytic Cracking
The fluid catalytic cracking (FCC) process is the heart of a modern refinery oriented toward maximum gasoline production. Within the entire refinery process, this process offers the greatest potential for increasing profitability; even a small improvement giving higher gasoline yields can result in a substantial economic gain. The FCC process increases the H/C ratio by carbon rejection in a continuous process and is used to convert the high-boiling, high-molecular-weight hydrocarbon fractions (typically, a blend of heavy straight-run gas oil, light vacuum gas oil, and heavy vacuum gas oil) to more valuable gasoline, olefinic gases, and other products.
The process consists of two main vessels: a reactor and a regenerator, which are interconnected to allow for transferring the spent catalyst from the reactor to the regenerator and the regenerated catalysts from the regenerator to the reactor. During catalytic cracking the feed is vaporized and the long- chain molecules are cracked into much shorter molecules by contacting the feed with a fluidized powdered catalyst at high temperature and moderate pressure.
Catalytic cracking reactions are believed to follow the carbonium ion mechanism, involving the following steps:
• Initiation: which starts from an early contact of an olefin with an active site of the catalyst at high temperature to produce the active complex corresponding to the formation of a carbocation
• Propagation: represented by the transfer of a hydride ion from a reactant molecule to an adsorbed carbenium ion
• Termination: corresponding to the desorption of the adsorbed carbe-nium ion to produce an olefin while the initial active site is restored
According to this mechanism, a catalyst promotes the removal of a negatively charged hydride ion from a paraffin compound or the addition of a positively charged proton (H+) to an olefin compound, which results in the formation of a carbonium ion. Carbonium ion is a positively charged molecule that has only a very short life as an intermediate compound and transfers the positive charge through the hydrocarbon. This carbonium transfer continues as hydrocarbon compounds come into contact with active sites on the surface of the catalyst that promote the continued addition of protons or the removal of hydride ions. The result is a weakening of carbon-carbon bonds in many of the hydrocarbon molecules and a consequent cracking into smaller compounds. These ions also react with other molecules, isomerize, and react with the catalyst to terminate a chain. Coke formation is unavoidable in the catalytic cracking process, which is probably formed by the dehydrogenation and condensation of polyaromatics and olefins. Fast deactivation by blocking the active pores of the catalyst is a consequence of coke deposition. During these reactions, the catalytic cracked gasoline produced contains large amounts of aromatics and branched compounds, which is beneficial for the gasoline’s octane level.
A typical modern FCC unit consists of the following steps (Figure 1.12):
• Preheating the feed and mixing with the recycle slurry oil from the bottom of the distillation column.
• Injecting the combined feed into the catalyst riser, where vaporization occurs.
Figure 1.12. Typical process scheme of a fluid catalytic cracking unit.
• Cracking the vaporized feed into smaller molecules by contact with the hot powdered catalyst coming from the regenerator.
• Separation of the cracked product vapors from the spent catalyst by flowing through a set of two-stage cyclones.
• Stripping the spent catalyst with steam to remove any hydrocarbon vapors before the spent catalyst returns to the regenerator.
• Regeneration of the spent catalyst to burn off the deposited coke with blown air. This reaction is exothermic and produces a large amount of heat, which is partially absorbed by the regenerated catalyst and provides the heat required for feed vaporization and the endothermic cracking reactions that take place in the catalyst riser.
• Passing the hot flue gas leaving the regenerator through multiple sets of cyclones that remove entrained catalyst from the flue gas.
• Suitably separating the cracked product vapors from the reactor from entrained catalyst particles by cyclone and sending them to the recovery section of the FCC unit to meet the product stream requirements.