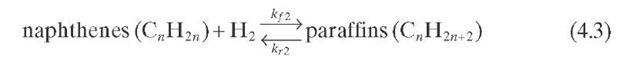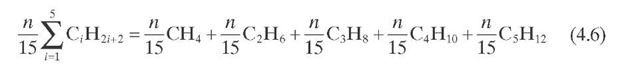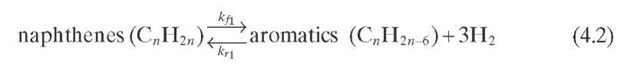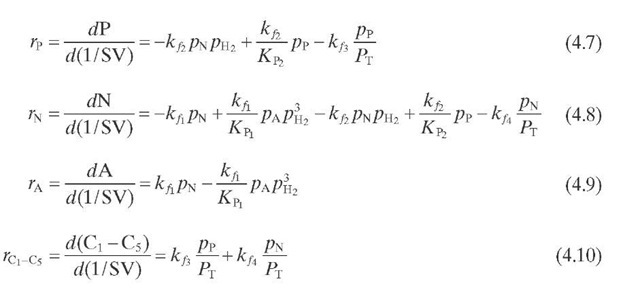Chemistry
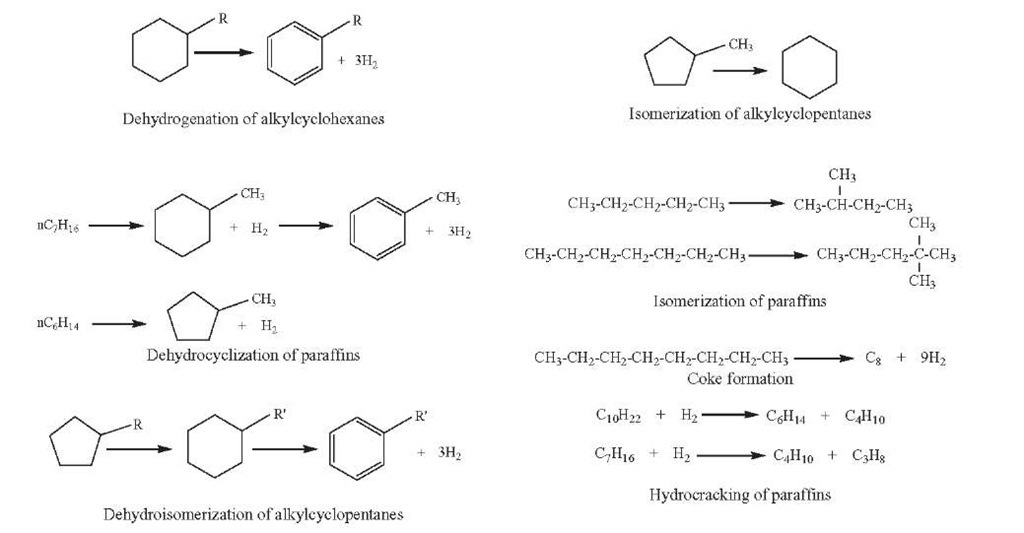
A large number of reactions occur in catalytic reforming over bifunctional catalysts, such as dehydrogenation and dehydroisomerization of naphthenes to aromatics, dehydrogenation of paraffins to olefins, dehydrocyclization of paraffins and olefins to aromatics, isomerization or hydroisomerization to isoparaffins, isomerization of alkylcyclopentanes and substituted aromatics, and hydrocracking of paraffins and naphthenes to lower hydrocarbons. All reactions are desirable except hydrocracking, which occurs to a greater extent at high temperature and converts valuable C+ molecules (reformate) into light gases. Some examples of these reactions are shown in Figure 4.2 – Most commonly, the reactions occurring during catalytic reforming are classified into the following four types.
Figure 4.2. Examples of catalytic reforming reactions.
Thermodynamics
The most rapid reactions (i.e., dehydrogenation of naphthenes) reach thermo-dynamic equilibrium, while the others are controlled by kinetics. Increasing the reaction temperature and lowering the pressure have both a positive effect on the reaction rate and thermodynamic feasibility as to the dehydrogenation of naphthenes (the most important reaction in catalytic reforming). The effect of these variables on thermodynamic equilibrium for the other reactions is slighter.
Table 4.2 summarizes the thermodynamic effect of the main reforming reactions. Other effects are the following:
• The dehydrogenation of naphthenes and paraffins is rapid and equilibrium concentrations are established in the initial portions of a catalyst bed.
• Olefins are readily hydrogenated, and at equilibrium only small concentrations can exist.
• The isomerization of paraffins is a sufficiently rapid reaction and primarily thermodynamically controlled, which means that actual concentrations are near equilibrium.
• The dehydrocyclization of paraffins is a much slower reaction and kineti-cally controlled.
• Hydrocracking rates increase with pressure and lower the reformate yield.
• Coking is very slow but increases rapidly at low hydrogen pressure and high temperature.
Thus, it is highly desired to operate reactors at high temperature and low pressure; however, catalyst deactivation due to coke deposition is also favored at those conditions. In addition, lowering hydrogen partial pressure results in an increase in the aromatization rate and a decrease in the rate of hydrocracking.
TABLE 4.2. General Thermodynamic Comparison of the Major Catalytic Reforming Reactions
|
Rate of Reaction |
Heat of Reaction |
Thermodynamic Equilibrium |
|
|
Naphthene dehydrogenation |
Very fast |
Very endothermic |
Reached |
|
Naphthene isomerization |
Fast |
Mildly exothermic |
Reached |
|
Paraffin isomerization |
Fast |
Mildly exothermic |
Reached |
|
Paraffin dehydrocyclization |
Slow |
Very endothermic |
Not reached |
|
Paraffin dehydrogenation |
Very fast |
Endothermic |
Not reached |
|
Hydrocracking |
Very slow |
Exothermic |
Not reached |
Kinetics
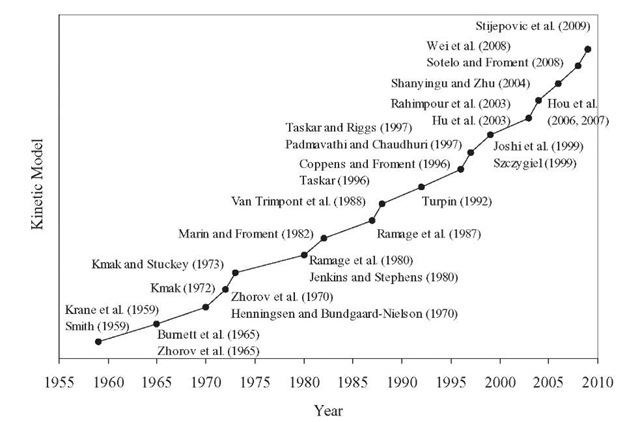
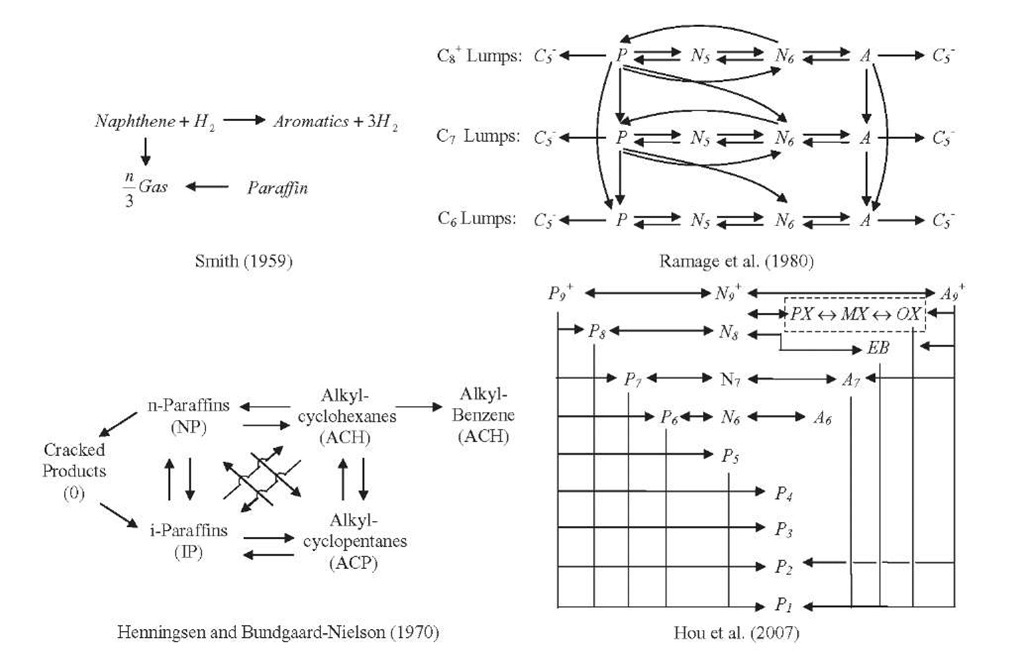
Various kinetic models for catalytic reforming reactions that have been reported in the literature have been the subject of several reviews. The level of sophistication of these models varies from a few lumps to detailed kinetic models and is related to the development of high-speed hardware and large-capacity computers. Since an exhaustive and critical review of the reported kinetic models in outside the scope of this topic, only a brief mention of the most relevant models will be made. The chronological evolution of the catalytic reforming kinetic modeling is presented in Figure 4.3.
The first attempts to model the kinetics of catalytic reforming reactions were reported more than 50 years ago. The oldest kinetic model, proposed by Smith (1959), divided naphtha feed into three types of hydrocarbons: paraffins, naphthenes, and aromatics. Each of these three hydrocarbon classes is represented by a single compound that has the average properties of that class. No distinction is made on the basis of the number of carbon atoms within each class. A kinetic analysis is developed which describes the reforming operation with satisfactory accuracy. Hydrogen and light gases (ethane, propane, and butane) are also taken into account in the model. This model thus involves five pseudocomponents: paraffins, naphthenes, aromatics, light gases, and hydrogen. This seems to be the first attempt to "delump" naphtha into various constituents. To simplify the catalytic reforming system, the following four reactions were considered:
Figure 4.3. Evolution of kinetic modeling for catalytic reforming.
Dehydrogenation of naphthenes to aromatics
Hydrogenation of naphthenes to paraffins
Hydrocracking of paraffins to lower hydrocarbons
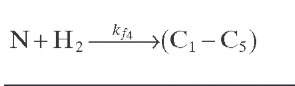
Hydrocracking of naphthenes to lower hydrocarbons
where
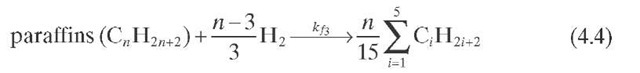
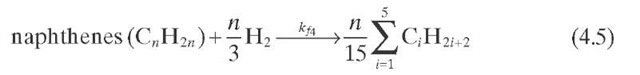
Reaction rate equations together with equilibrium and rate constants (kfi for the forward reaction and kri for the reverse reaction) as a function of temperature (T), partial pressure (p ), total pressure (PT), and the inverse of space velocity (1/SV) are summarized in Table 4.3 – From this table the following mass balance for paraffins (P), naphthenes (N), aromatics (A), and light ends (C1- C5) can be derived:
TABLE 4.3. Kinetic Equations of the Smith (1959) Modela
|
Reaction |
Reaction Rate |
Equilibrium |
Constant, KP |
Rate Constant for the Forward Reaction kf |
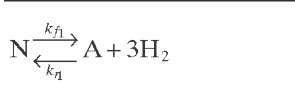 |
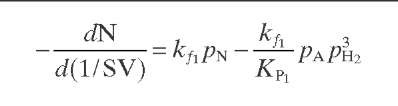 |
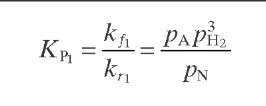 |
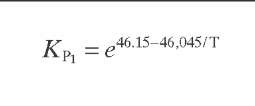 |
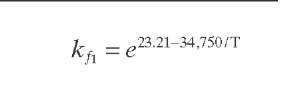 |
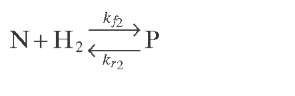 |
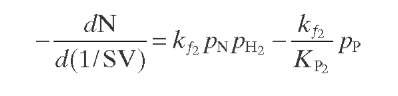 |
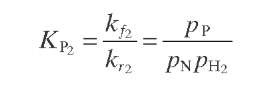 |
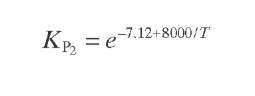 |
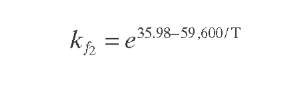 |
 |
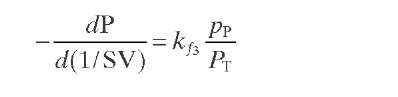 |
 |
 |
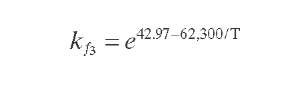 |
 |
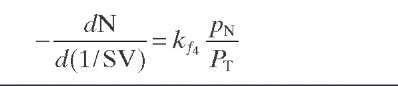 |
 |
 |
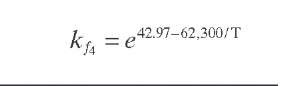 |
Krane et al. (1959) proposed another more extensive attempt to model catalytic reforming reactions of whole naphtha, which consisted of a reaction network of 20 pseudocomponents with hydrocarbons ranging from 6 to 10 carbon atoms, as well as the difference between paraffins, naphthenes, and aromatics within each carbon number group, which undergo 53 reaction steps. Krane’s proposed reaction network can be summarized as follows:
|
Paraffins |
||
 |
(4.11) |
|
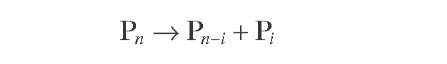 |
(4.12) |
|
|
Naphthenes |
 |
|
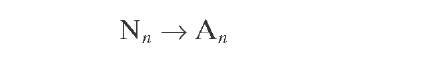 |
(4.13) |
|
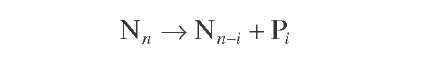 |
(4.14) |
|
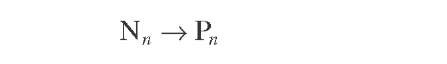 |
(4.15) |
|
|
Aromatics |
 |
|
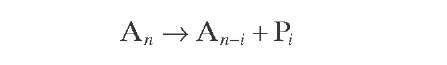 |
(4.16) |
|
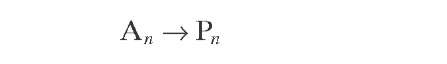 |
(4.17) |
|
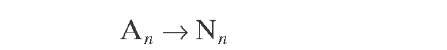 |
(4.18) |
All reactions are represented by a pseudo- first- order rate equation with respect to hydrocarbon concentration. Reaction rate constants were derived from experiments with whole naphtha. More details about the reaction rate equations of the Krane et al. (1959) model will be given in later sections of this topic, together with various improvements on it.
Diverse modifications and applications of these two pioneer works have been reported in scientific papers. For example, Smith’ s model was modified by Vinas et al. (1996) to include discrimination between the reaction rates for aromatization of five- and six-ring naphthenes, two types of paraffins with different reactivities, and an overall hydrodealkylation reaction. Bommannan et al. (1989) estimated the values of activation energies from two sets of plant data using Smith’s model. Dorozhov (1971) made a distinction between paraffins C5-C6 and paraffin C7 in order to improve the model, which became more complicated, and its predictability capacity was only slightly better. Moharir et al. (1979) incorporated a deactivation function for both acidic and metallic functions of the catalyst into Smith’s model, in order to simulate and optimize a naphtha catalytic reforming plant.
Lee et al. (1997) and Lid and Skogestad (2008) modeled a catalytic naphtha reformer with continuous catalyst regeneration using Smith’s model with the goal of determining optimal operating conditions. In the work of Lid and Skogestad (2008) – the process model is fitted to 21 data sets collected in a two-year period from a commercial naphtha reformer. More recently, Liang et al. (2005) used Smith’ s model to develop a physical model to simulate a naphtha catalytic reforming radial flow reactor unit with four reactors in series. Kinetics and thermodynamics equations were selected to describe the reforming reactions based on idealizing the complex naphtha mixture by representing the paraffin, naphthene, and aromatic groups by single compounds.
The kinetic parameter values were estimated using experimental information obtained in a bench-scale fixed-bed reactor. This model was incorporated in a fixed-bed one-dimensional pseudohomogeneous adiabatic reactor model.
Burnett et al. (1965) proposed a pseudo-first-order kinetic model involving only hydrocarbons with seven atoms of carbon. Zhorov et al. (1965, 1970) incorporated the relationship between the reaction rate constants and the composition of the naphtha feed in a kinetic model consisting of C5 and C6 lumps and direct formation of aromatics from paraffins. Henningsen and Bundgaard-Nielson (1970) proposed a different treatment for the C5 and C6 ring naphthenes and expressed the reaction rate constants in the form of an Arrhenius-type equation to account for the influence of temperature. Catalyst deactivation was also included in the model. Values for the heat of reaction and activation energies were also provided. Kmak (1972) described a model that incorporated the naphtha catalytic reforming reactions with Hougen-Watson-Langmuir-Hinshelwood kinetics, in which the rate equations account explicitly for the interaction of chemical species with the catalyst. Later, Kmak and Stuckey (1973) used pure components, mixtures, and naphtha feed to develop a detailed catalytic reforming kinetic model over a wide range of reaction conditions. They used this model to simulate the power-forming process within a wide range of operating conditions. The model was capable of determining the concentration profiles of 22 components in four reactors in series.
Ramage et al. (1980, 1987) developed a detailed kinetic model based on extensive studies with pure components and a narrow-boiling fraction of naph-thenes in a pilot-plant reactor. The kinetic model involves a reasonable number of lumps and pathways, captures the reactivity differences between particular feeds, and incorporates catalyst deactivation by coke formation. The model considered C6- C8 lumps of naphthenes, paraffins, and aromatics and was able to predict interactions between 13 lumps that undergo reactions of hydro-cracking, hydrogenation-dehydrogenation, cyclization, and isomerization. Reversible reactions were assumed for hydrocarbons with equal carbon-atom numbers, and irreversible reactions for those between hydrocarbons with different numbers of carbon atoms. Jenkins and Stephens (1980) employed first-order rate equations, including reversible ones, to develop a kinetic model with 78 reactions involving 31 components. The effect of pressure on the reaction rates was simulated by means of a pressure factor with a characteristic exponent for each particular reaction.
Marin and Froment ( 1982) developed a kinetic model for the catalytic reforming of naphtha by first studying C6 reforming and then C7 reforming (Van Trimpont et al-, 1988). The model considered 5 to 10 carbon atoms and a reaction network including 23 pseudocomponents and used Hougen-Watson rate equations. Turpin (1992) combined fractionation modeling with kinetic modeling of the reforming processing to determine how best to meet processing requirements associated with the benzene content in reformulated gasoline.
Taskar (1996) and Taskar and Riggs (1997) employed a rigorous kinetic model to optimize the performance of an industrial catalytic reforming plant by studying operating modes and the influence of operational variables. They develop a more detailed kinetic model involving 35 pseudocomponents. Coppens and Froment (1996) improved catalytic reforming models by including diffusional effects in the rate equations. In the model proposed the porous nature of the catalyst support is approximated by a self-similar fractal structure.
Padmavathi and Chaudhuri (1997) developed a simulation model to monitor the performance of a commercial plant, in which details were given as to how the feed and the reacting scheme were lumped, as well as details on parameter estimation and model validation. They proposed a lumped kinetic model with 26 pseudocomponents.
Szczygiel (1999) investigated the kinetics of catalytic reforming by making use of pure components as feed. He reported an algorithm to optimize the porous structure of the reforming catalyst, consisting of three major steps: (1) analysis of kinetic phenomena in the catalyst grain, (2) analysis of diffusion phenomena in the catalyst grain, and (3) construction of a mathematical model to optimize the parameter values for the porous structure of the reforming catalyst grain. For the kinetic analysis, the paraffin reaction paths and the kinetic scheme are determined based on experiments in a flow – through catalytic reactor. It is proposed that the kinetic model be used to optimize the catalyst pore structure.
Joshi et al. (1999) proposed a rigorous pathway-level approach for modeling catalyst reforming consisting of 79 components with 464 reactions. Rahimpour et al. (2003) presented a kinetic and deactivation model for the simulation of an industrial naphtha catalytic reforming unit. Hu et al. (2003) reported a kinetic model for catalytic reforming with 17 lumps and 17 reactions. The adsorption and chemical lumps on the catalyst surface and deactivation of catalyst due to coking are also taken into account. Later, Hou et al. (2006, 2007) subdivided the eight-carbon aromatics lump of the Hu et al. (2003) model into their four isomeric compounds: PX (para-xylene), MX (meta-xylene), OX (ortho-xylene), and EB (ethylbenzene). Stijepovic et al. (2009) developed a general framework for modeling the catalytic reforming process. A semiempirical kinetic model was proposed consisting of 18 lumps based on paraffins, i-paraffins, naphthenes, and aromatics. Different values of activation energies were considered for each reaction. The model parameters were estimated by benchmarking with industrial data. The model is able to predict the concentration of hydrogen and light gases. Shanyinghu and Zhu (2004) presented a model involving several reactions to illustrate molecular modeling of the naphtha reforming process. Figure 4.4 shows reaction schemes used in the development of some of the kinetic models described above. The rate equations, values of kinetic parameters, properties of the catalyst and feedstock used during experiments, and other details may be found in the respective references.
Figure 4.4. Examples of some reaction schemes used to develop catalytic reforming kinetic models.
More recently, Wei et al. (2008) developed an approach to modeling the reaction kinetics of the catalytic reforming by introducing a number of representative pseudocomponents by Monte Carlo simulation. By this means the complexity of the feed was reduced and a reaction network of this synthetic feed was generated by computer using graph theory. Sotelo and Froment (2009) introduced a fundamental kinetic model for the catalytic reforming process. The model is based on the fundamental chemistry occurring on both the acid and metal sites of a Pt-Sn/Al2 O3 catalyst. The single – event concept was applied in the development of rate expressions for the elementary steps on the acid sites. The kinetic model was used in pseudohomogeneous and heterogeneous reactor models for the simulation of a commercial adiabatic catalytic reforming unit with three reactors in series with centripetal radial flow.
As a summary of the state of the art in kinetic modeling for naphtha catalytic reforming, it can be observed that on the one hand, most published kinetic models based on the lumping approach report the rate constants to be dependent on feed and catalyst properties. Some models are not capable of predicting the composition of alkylcyclopentanes, the composition of n-paraffins and /-paraffins, the detailed composition of hydrocracking reaction products, nor the entire range of hydrocarbons present in naphtha composition. The level of sophistication varies from just a few lumps to a very detailed kinetic model. On the other hand, sophisticated models based on fundamental approaches (e.g., a single-event kinetic model), although overcoming some drawbacks of the lumping models still have to be validated under conditions (e.g., other feedstocks) different from those under which they were derived, and provide a more convincing comparison with industrial reality. Also, lumping models involve a reduced number of kinetic parameters and require relatively small amounts of experimental data for their estimation, whereas fundamental detailed models are quite complicated, with a large number of parameters, and frequently need more experiments. Therefore, a kinetic modeler faces a considerable dilemma: One uses either a lumped-kinetic model or a more fundamental approach. The decision is not an easy one to make. However, there are some important points that affect this decision. Most of the time, the model needs to simulate a commercial unit and anticipate the effect on product yield and quality of minor changes in process parameters. If a model with only a few lumps is chosen, the predictive capability surely is not sufficient to represent the desired situation. But if a detailed mechanistic model is selected, it may be too complex to implement, not because of the solution of the model, which with modern computers and algorithms has become a relatively easy task, but due to the cost and amount of experimental information needed to determine the model parameters. Thus, why not use an intermediate approach that maintains the simplicity of the lumping approach and is detailed enough to correctly predict the behavior of a commercial catalytic reforming plant? By an intermediate approach we mean that the number of lumps is such that the composition of the product is predicted with all the components desired. The answer to this question is the reason that lumped kinetic models are still commonly used to characterize reactive groups and to describe the reaction kinetics of complex processes in a tractable manner. It is the general conclusion of all the published scientific papers that the lumping approach is sufficiently reliable in describing the relationships between process variables and reaction rates. Comparisons of simulated results using lumping kinetic models with data obtained at different scales, including those on a commercial scale, support this conclusion.
Catalysts
Catalytic reforming reactions are conducted in the presence of hydrogen over hydrogenation-dehydrogenation catalysts. The dual function of the reforming catalysts is provided by (1) the acid centers of the support (alumina or silica-alumina), and (2) the metallic centers (platinum with other metals dispersed on the support, e.g., rhenium). For maximum catalyst efficiency, a proper balance between the acidic and dehydrogenating functions must be achieved. The activity of platinum is inhibited by sulfur, which adsorbs revers-ibly on the platinum crystallites. That is why the catalytic reforming feed needs to be hydrotreated to lower its sulfur content to <1 wppm. Water must also be kept at a low content to avoid leaching of chloride and thus loss of acid strength.
In addition to Pt, modern multimetallic catalysts contain highly dispersed rhenium (Re) and in some cases tin (Sn). In fact, two catalyst formulations prevail commercially: Pt-Re/Al2O3 and Pt-Sn/Al2O3. Pt-Re catalyst is the most stable and is preferred in semiregenerative units, while Pt-Sn has the highest selectivity at low pressure and is the best choice for continuous regeneration reforming units. /-Alumina is the most common reforming catalyst support. The platinum must be dispersed over the alumina surface such that the maximum number of active sites for dehydrogenation is available. To achieve the appropriate acidic level of the catalyst, chloride is added prior to use.
The noble metals (platinum and rhenium) are considered to be catalytic sites for the dehydrogenation and hydrogenolysis reactions, and chlorinated alumina provides the acid sites needed for isomerization, cyclization, and hydrocracking reactions. Acid-catalyzed reactions together with the Pt-catalyzed dehydrogenation function are largely responsible for hydroisomer-ization reactions that lead to the formation of aromatics. Paraffins may be isomerized over the acidic function of the catalyst to provide higher-octane branched paraffins. Another acid-catalyzed reaction is paraffin hydrocracking to produce lighter products. Paraffins also undergo cyclization to cyclohexanes, which is believed to proceed through an olefin intermediate, produced by Pt-catalyzed dehydrogenation. The cyclization of the olefin may be catalyzed by the alumina support. During normal operation, the activity of the catalyst is reduced over time by coke deposition. Coking results from secondary reactions of the hydrocarbons, particularly olefins. The catalyst activity can be restored periodically by regeneration (coke burn- off) at high temperature. Coke burning is usually done with air at 400 to 500°C. Normally, the catalyst can be regenerated three or four times. The catalysts used in semiregenerative units must have long catalyst life cycles. In continuous regeneration units, the catalyst flows through the reactors and is regenerated continuously in a separate regeneration vessel that is part of the reactor-regenerator loop.
In semiregenerative units, to compensate for catalyst deactivation, the reactor temperature is increased constantly until the maximum allowable value is reached, or the selectivity to desired products is too much reduced, or the octane of the liquid product is declining. When this happens, the catalytic reforming unit requires a shutdown for regeneration every 6 to 12 months. This relatively long cycle can be obtained by operating under milder conditions of high partial pressure of hydrogen, lower- reactor temperatures, and lower- octane products.
In continuous regeneration units, portions of the catalyst are continually being regenerated outside the process and returned to the reactors so that high selectivity and activity are maintained. Continuous units operate under severe conditions to yield high-octane high-aromatics production, at low hydrogen partial pressures and higher reactor temperatures.
The catalysts are different depending on the type of reforming unit. For semiregenerative units the catalysts contain platinum or platinum modified by rhenium or, to a lesser extent, iridium. The support is most often /-alumina, although some catalysts are supported on ^-alumina. Rhenium or iridium is used to enhance the life of the catalyst. There are two main shapes of catalysts, cylindrical and spherical. The density of the catalysts varies in the range 0.5 to 0.8g/cm3- For continuous regeneration units, spherical catalysts are used to transport catalyst from the reactors to the regenerator, and back. The use of spherical catalysts rather than extrudate is to avoid catalyst dusting and breakage. In continuous regeneration units the catalyst is circulated at a rate of about one regeneration per week. Platinum and tin on – alumina are the typical catalysts used in these units. The tin is used to reduce the hydrogenoly-sis activity of the platinum and to improve reformate yields. There are reports in the literature of new catalysts used to increase yields of reformate octane and to reduce coke production.