KEY CONCEPTS:
Upon completion of this topic, it is expected that the reader will understand these following concepts:
• The formation of acid from respiratory and metabolic mechanisms and physiological pH
• Three important chemical buffers found in the bloodstream
• Respiratory and renal compensation by deriving the acid-bicarbonate formula
• Acidosis as an imbalance
CASE STUDY:
The Paramedics were called to the local community college for an instructor suffering an acute asthma attack. Mr. Byrnes had a lengthy history of asthma beginning in high school. His last attack, four months ago, had required intubation and two days on ventilatory support.
The nurse in the health office had placed Mr. Byrnes on oxygen along with monitoring pulse oximetry. He had tried to use his rescue inhaler but could not take deep enough breaths. Even though his pulse oximetry reading was at 97%, one look indicated that he was laboring, tachypneic, and fearful.
OVERVIEW
Ventilation is more than just the movement of air in and out the lungs. For the Paramedic, examining ventilation involves understanding the physiology of gas exchange and the different ways the body maintains a balanced pH. Metabolically, the body’s organs work together to maintain an acid-base balance. This topic examines the basic chemistry of pH, the chemical buffers, and how ventilation provides a first line of defense from acid imbalance. Respiratory and renal mechanisms also play an important role by driving the acid-bicarbonate formula, which works to regulate pH. Respiratory acidosis is seen as an imbalance that the Paramedic can recognize and treat.
Respiration and Oxygen Transportation
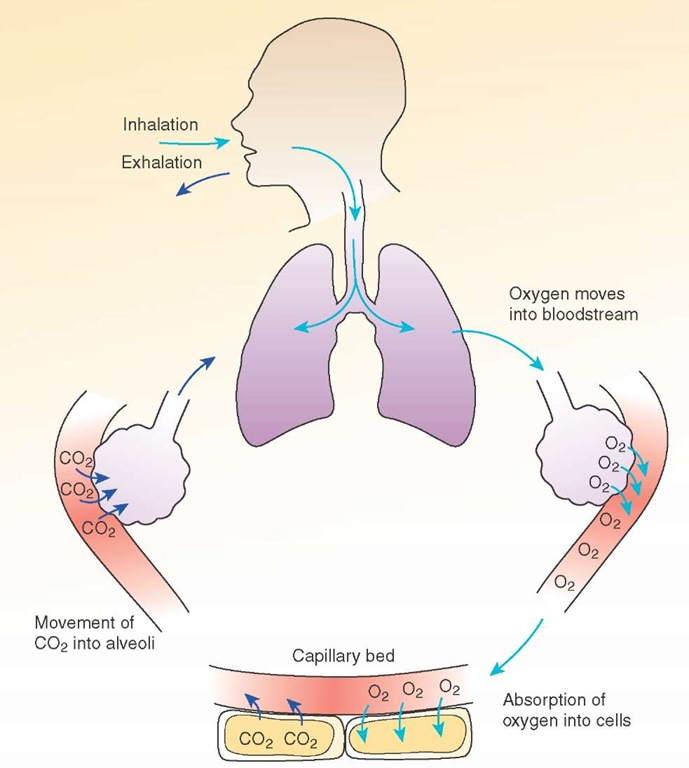
The term "respiration" has several definitions applicable to Paramedic practice.1 The first definition of respiration is the movement of respiratory gasses in and out of the lungs.On a cellular level, respiration is defined as "the chemical processes by which an organism supplies its cells and tissues with the oxygen needed for metabolism and relieves them of the carbon dioxide formed in energy-producing reactions." Respiration includes everything from inspiration, movement of oxygen into the bloodstream, absorption of oxygen into the cells, utilization of oxygen to make energy, the movement of carbon dioxide to the bloodstream and ultimately across into the air in the alveoli, and the exhalation of carbon dioxide into the atmosphere (Figure 25-1). Through this process, the human body is able to live and function.
The components of this process that the Paramedic can affect during the breathing step in the resuscitation involve several actions that improve oxygenation and ventilation. Before we can assess those components, let’s review how the body transports oxygen and carbon dioxide.
Oxygen Transport
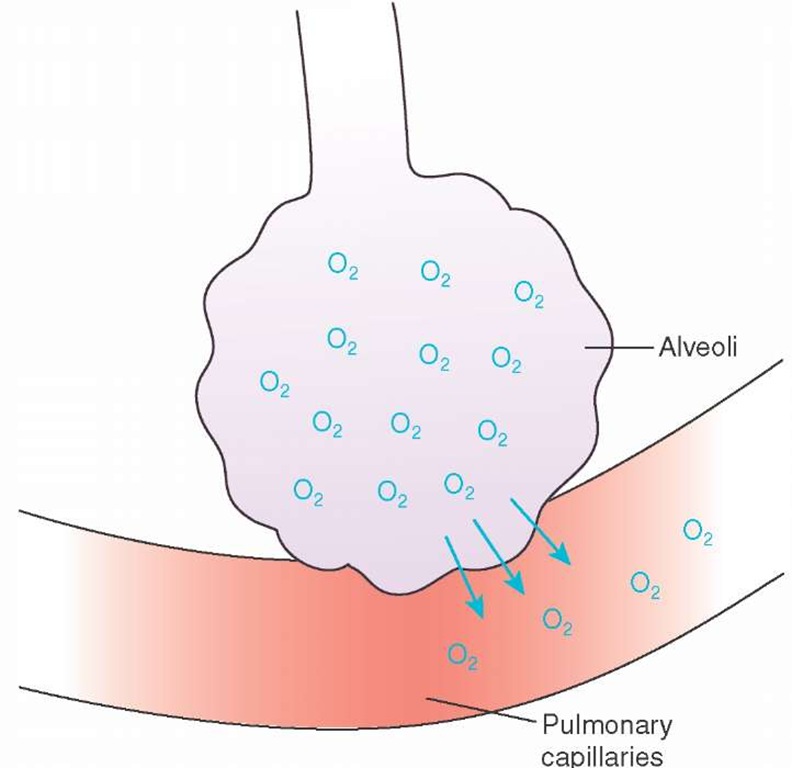
Oxygen comprises 21% of room air.Inspiration fills the lungs with oxygen-rich air. Oxygen diffuses across the alveoli and capillary wall because of the higher concentration of oxygen in the alveoli compared with the lower concentration of oxygen in the blood surrounding the alveoli (Figure 25-2).
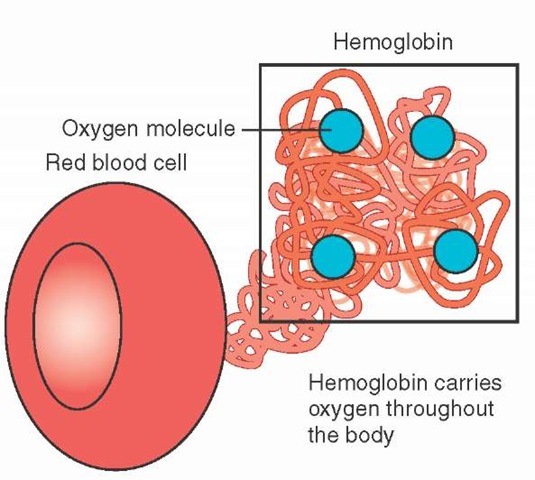
Oxygen is transported to the tissues by two different mechanisms. Approximately 3% of oxygen that enters the bloodstream is dissolved into the plasma, or liquid portion of the blood, similar to the way carbon dioxide is dissolved into liquid to produce the fizz in carbonated beverages. This is an ineffective method for carrying oxygen to the tissues because oxygen does not easily dissolve into liquid and only a small amount of oxygen can be delivered. A more effective mechanism for oxygen transport involves binding the oxygen molecules directly to a compound or structure within the blood for transportation to the tissues. This transport mechanism is responsible for the other 97% of oxygen transported to the tissues and involves red blood cells and the hemoglobin molecule that makes up the majority of the red blood cell.
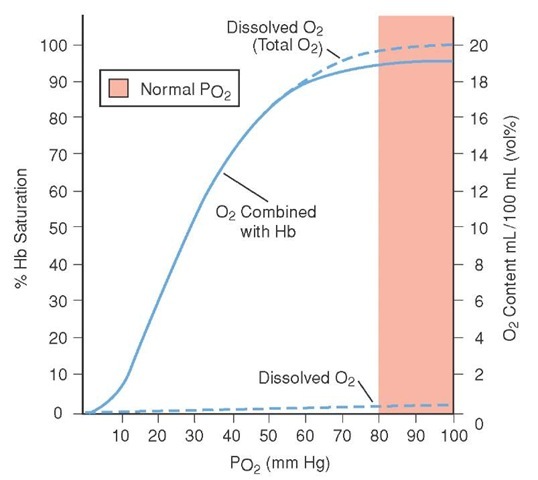
Each red blood cell contains approximately 270 million hemoglobin molecules. Each hemoglobin molecule can normally bind up to four oxygen molecules (Figure 25-3). When the hemoglobin molecule attaches at least one oxygen molecule, it is called oxyhemoglobin. The hemoglobin molecule that is not attached to any oxygen molecules is called deoxyhemoglobin. As oxygen attaches to each of the binding sites, the shape of the hemoglobin molecule opens up around the other oxygen-binding sites, making it easier to bind oxygen to the next site. Due to this property, it takes a smaller increase in partial pressure of oxygen to saturate the hemoglobin molecules with oxygen when hemoglobin is in the deoxyhe-moglobin state. As hemoglobin nears complete saturation, it then takes a larger change in partial pressure of oxygen to fully saturate all oxygen-binding sites. Figure 25-4 demonstrates this relationship between partial pressure of oxygen and hemoglobin saturation. It should be noted that arterial blood typically has a saturation of approximately 97%, whereas venous blood will typically have a oxygen saturation of approximately 75%.2
There are several factors that affect oxygen’s ability to bind to hemoglobin. Some of these factors are used by the body to enhance the release of oxygen as part of normal transport, whereas others occur when the body is ill or injured (Table 25-1). The factors that increase oxygen binding in effect shift the curve in Figure 25-4 to the left. By shifting the curve to the left, it takes a smaller change in partial pressure of oxygen to increase the saturation of the hemoglobin. Conversely, the factors that decrease oxygen binding move the curve to the right, requiring a larger change in partial pressure of oxygen to saturate the hemoglobin molecules.
Figure 25-1 The process of respiration.
Figure 25-2 Oxygen movement across the alveoli-capillary membrane.
Figure 25-3 Red blood cells contain hemoglobin, the oxygen-carrying portion of the blood, which can transport up to four oxygen molecules per hemoglobin molecule.
Figure 25-4 The oxygen-hemoglobin dissociation curve. In the lower portion of the curve, it takes a small increase in partial pressure of oxygen to produce a large increase in oxygen saturation.
Table 25-1 Factors That Affect the Ability to Bind Oxygen to Hemoglobin
|
Increased |
Decreased |
|
• Alkalosis |
• Acidosis |
|
• Decreased CO2 |
• Increased CO2 |
|
• Decreased temperature |
• Increased temperature |
|
• Decreased 2,3-BPG |
• Increased 2,3-BPG |
Both alkalosis and acidosis will be discussed later in this topic. Alkalosis will cause a shift in the curve to the left, increasing the affinity of hemoglobin to oxygen, and acidosis will cause a shift in the curve to the right, decreasing the affinity of hemoglobin for oxygen. This change in affinity helps the hemoglobin either hold on to the oxygen molecules or enhances the release of oxygen molecules from hemoglobin.
A second way the body enhances release of oxygen from hemoglobin is through a compound called 2,3-BPG. 2,3-BPG occurs naturally in the hemoglobin, enhancing the release of oxygen from hemoglobin at the tissues to provide oxygen for the energy production process at the cellular level. This compound can be found in higher concentration in situations where the amount of oxygen inhaled into the lungs is decreased or in conditions where there is chronic tissue hypoxia. This is a way for the body to compensate for less available oxygen at high altitudes or for advanced chronic respiratory conditions. Increased production of 2,3-BPG helps to prevent hypoxia in the tissues.
Temperature also affects hemoglobin’s ability to off-load oxygen at the tissues. Decreased temperatures shift the curve to the left while increased temperatures will shift the curve to the right. This is why patients who have sustained traumatic injury, who are often cold from exposure, even in warmer climates, should be kept warm and provided with supplemental oxygen to compensate for the difficulty in off-loading oxygen as a result of decreased body temperature.
Once the hemoglobin circulates to the tissues, some of the oxygen bound to hemoglobin dissociates, or is released, from the hemoglobin into the blood and taken into the cells. The environment in the capillary blood has a lot of the factors that enhance oxygen release from hemoglobin, including increased carbon dioxide and increased levels of 2,3-BPG. In the cell, oxygen is used with glucose to produce energy to carry out the cell’s functions (e.g., contraction for muscle cells, chemical production for an endocrine cell, or to fight bacterial invaders).
Carbon Dioxide Transport
Carbon dioxide is a by-product of cellular respiration. During the chemical processes within the cell, oxygen and glucose are used to produce energy for the cell. Carbon dioxide and water are produced as waste products of this reaction. The carbon dioxide diffuses across the cell membrane and into the blood. Once in the blood, some carbon dioxide is taken up and carried to the lungs in the red blood cells while some stays dissolved in the plasma.
Carbon dioxide is 20 times more soluble than oxygen in the blood at the same partial pressure, and much of the carbon dioxide transported by the blood is dissolved within the plasma. Some of the dissolved carbon dioxide is then absorbed into the red blood cells and then released back into the blood as bicarbonate, which is then transported to the lungs. A small amount of carbon dioxide travels back to the lungs attached to the hemoglobin molecules, at a different site from the oxygen molecules, and some carbon dioxide molecules combine with other compounds in the blood. Therefore, carbon dioxide is transported to the lungs in one of three ways: dissolved in the blood plasma, attached to hemoglobin, or contained with bicarbonate.
At the lungs, the bicarbonate is changed back to carbon dioxide and water. The dissolved carbon dioxide off-gasses and the hemoglobin releases its carbon dioxide. The carbon dioxide then diffuses across the alveolar membrane and into the alveolar air, ready for exhalation. The transport of carbon dioxide is affected by many factors including both the amount of carbon dioxide produced by the cells (metabolism rate) and the amount of blood volume circulated through the lungs. If the patient is hypotensive, there is less blood circulating through the lungs and reduced transportation of carbon dioxide to the lungs. Similarly, if the patient has hem-orrhaged than there are fewer red blood cells, which means less hemoglobin to bind oxygen and plasma to carry the carbon dioxide.
These factors culminate to increase carbon dioxide levels in the tissues. Carbon dioxide, together with water, forms a weak acid called carbonic acid (H2CO3). The level of carbonic acid in the body is referred to as the "acid load."
During any form of shock there is an increase in the body’s acid load. An increased acid level in the body can have devastating effects to the normal metabolic functions of the body if not corrected.
Acid-Base Balance
Acids are created in the course of both aerobic (with oxygen) and anaerobic (without oxygen) metabolism. The majority of acid in the body is formed when excess carbon dioxide reacts with water to form carbonic acid (H2CO3) before conversion into bicarbonate. It is called the respiratory acid as it is the intermediary step in carbon dioxide transport. Other acids (e.g., lactic acid and pyruvic acid formed during anaerobic metabolism, and amino acids formed by the breakdown/ oxidation of proteins) are called the metabolic acids. Regardless of the source, an overabundance of acid can interfere with the normal enzyme action within the cells.Acid levels must be controlled by the body.
An acid, by definition, is a molecule that has a proton (a positively charged atomic particle) that is not orbited by a paired negatively charged atomic particle called an electron. The particle that exists in nature with one proton is the hydrogen particle and thus hydrogen is considered a primary acid. And acids are chemical compounds with positively charged hydrogen (H+) particles or ions attached; ions being charged particles. Being positively charged, these hydrogen ions are extremely reactive, meaning that the hydrogen ion wants to couple with other molecules, even if it means uncoupling one chemical molecule from another and bringing about the destruction of the molecule compound in order to allow the hydrogen to attach, or bond, with another chemical.
In some cases, a molecule may be negatively charged, such as the hydroxyl molecule (—OH). These chemicals are called bases and bases lack a proton and want to accept the protons from acid in order to become electrically balanced. For example, if an acid (H+) was to join with a base (—OH), the result would be water (H2O). The acid is then said to be buffered, or rendered neutral (i.e., not having an electrical charge) once it combined with a base to form water.
Trying to describe the different amounts of acidity or alkalinity in a solution can be difficult, since there can be as much as a thousand-fold difference from one extreme to the other. Practically speaking, using such a range is difficult. To ease the process of describing the strengths of acids and bases, the pH scale (abbreviated for potential hydrogen) was developed to describe the differing degrees of acidity or alkalinity. Mathematically, pH is the negative logarithm of the hydrogen ion concentration (pH = —log10[H+]). The range of pH is from 0 to 14, with 7 being neutral; pure distilled water is neutral. A weak acid has a pH closer to 7, somewhere in the range of 5 to 7, whereas a weak base has a pH somewhere in the 7 to 9 range. Human blood has a pH of 7.35 to 7.45, and is slightly alkaline. When the concentration of hydrogen ions increases, the solution becomes more acidic, and the pH decreases. When the concentration of hydrogen ions decreases, the solution becomes more basic, and the pH increases (Figure 25-5).
Excessive amounts of acid in the tissues, in sum total called an acid load, can be devastating to proteins within the cells. Excess acid can eventually break down (i.e., denature) proteins in the cells and eventually lead to cell death or necrosis.
Acid is eliminated from the interstitial space when the acid dilates the capillary beds. The acid passes into the capillary bed and the acid is washed out in the blood. Upon entering the bloodstream, the acid can now be acted upon by the body’s buffering mechanisms.