KEY CONCEPTS:
Upon completion of this topic, it is expected that the reader will understand these following concepts:
• Paramedic use of 12-lead ECG as the first step in a critical care pathway for patients with acute coronary syndrome
• Accurate acquisition of the 12-lead ECG
• Analysis of the 12-lead ECG to make a patient prognosis, determine treatment, and plan for an appropriate destination
Case study:
The Paramedics were called to the home of Jennie Swinter. Mrs. Swinter is an 82-year-old widow, living alone on a small farm that she still farms for vegetables. She called EMS because she became exhausted and out of breath after walking to the bathroom. The inexperienced Paramedic commented that at her age, Mrs. Swinter should be tired and out of breath. The more experienced Paramedic suggested that many acute processes could account for Mrs. Swinter’s complaints.
The Paramedics obtained an ECG right after placing Mrs. Swinter on oxygen. It showed ST elevation and hyperacute T waves in Leads II, III, and aVF. Mrs. Swinter complained of feeling very lightheaded and afraid. Repeat vital signs were obtained. Rather than the 118/66 found earlier, she had a pressure of 80/48. She also had jugular venous distention while semi-sitting and clear lung sounds.
OVERVIEW
Death from acute myocardial infarction remains a leading reason for mortality in the United States despite advances in medicine. It has been estimated that 50% of patients with acute coronary syndrome (which, if left untreated, leads to AMI) are transported by EMS and the majority of cardiac arrests occur in the prehospital setting. It is therefore important that Paramedics be able to identify and aggressively treat these patients.
Chief Concern
There are a number of causes of chest pain. Of particular concern for the Paramedic is chest pain of a cardiac etiology. Patients with cardiac-related chest pain are at high risk for acute myocardial infarction and sudden cardiac death.
The identification of the patient with potential for acute myocardial infarction is predicated on a clinical history which is suggestive of acute coronary syndrome and electrocardio-graphic findings. The latter, electrocardiographic findings, are not always present in patients who are at the beginning of the event (i.e., early in the evolution of the acute myocardial infarction). The maxim "Treat the patient, not the monitor" holds true for these patients. Treatment for suspected acute myocardial infarction—specifically morphine, oxygen, nitrates, and aspirin—should not be withheld because of a lack of electrocardiographic findings. It has been estimated that upward of 50% of patients who will develop an acute myocardial infarction had no confirmatory electrocardiographic (ECG) findings upon the initial ECG.1-4
STREET SMART
One study suggested that serial 12-lead ECGs identified acute myocardial infarctions in 75% of patients upon whom the initial 12-lead ECG exhibited nonspecific ECG changes. The importance of an early baseline 12-lead ECG was supported by that study. Therefore, it is advisable for all Paramedics to obtain an initial baseline 12-lead ECG as soon as possible. Thereafter, serial 12-lead ECGs should be performed every 15 to 30 minutes for the first 2 hours of patient contact.
However, that is not to dismiss the importance of obtaining a 12-lead ECG as soon as possible on a patient with suspected acute coronary syndrome. A 12-lead ECG can also help the Paramedic estimate the location of the coronary occlusion, ascertain the ventricular wall involved, and predict the coronary artery that is affected. Not only is this information valuable downstream, to the emergency physicians and cardiologists who will eventually treat the patient, but it is important for the Paramedic. By being able to estimate the location and extent of injury, the Paramedic can predict, with relative confidence, the clinical course that the patient will take. This prognostic ability permits the Paramedic to prepare for predictable complications related to the acute coronary event.
Atypical Presentations of ACS
While it is obvious that patients with substernal chest pain (SSCP) should have a 12-lead ECG, other occasions when a 12-lead ECG may be necessary are sometimes less obvious. For example, middle-aged females often do not present with chest pain. These patients tend to present with atypical presentations for acute coronary syndrome.5,6
These atypical presentations can include sharp rather than crushing chest pain, shortness of breath, unexplained weakness, and sudden diaphoresis. Unfortunately, some of these symptoms can be mistaken for menopausal signs, leading to delayed treatment. The belief that a patient with an acute coronary event must have concomitant substernal chest pain is a misconception.
Another group of patients who have an atypical presentation are the elderly. This group of patients tends to have a higher frequency of acute myocardial infarction, even when identified early in the evolution, and complications such as heart and renal failure.
For these reasons the Paramedic should have a low threshold for 12-lead ECG acquisitions. The best support for this argument is found in the frequency that 12-lead ECG is obtained in the emergency department.
Rhythm Strip
The primary mission of all Paramedics has always been to prevent sudden cardiac death from dysrhythmia. This has been the essence of Paramedic care since the advent of Dr. Pantridge’s "mobile coronary care units" in Belfast, Ireland, over half a century ago. However, with the advent of fibrinolytics and invasive cardiology, the original mission has been expanded to include rapid acquisition and interpretation of 12-lead ECGs.
The 12-lead ECG stands at the center of the decision pathway when managing patients with ischemic chest pain. Delays in obtaining the 12-lead ECG must be eliminated whenever possible. The most effective means of obtaining a 12-lead ECG at the earliest point in time is to have a Paramedic obtain one.7
The importance of obtaining a rapid and accurate 12-lead ECG is underscored by the American Heart Association’s (AHA) statements. The AHA states that upon recognition of acute coronary syndrome and a suspected acute coronary event, a 12-lead ECG should be obtained as soon as possible but no later than ten (10) minutes upon arrival at the hospital.8,9 This rapid 12-lead ECG acquisition and interpretation will facilitate the patient’s transfer to cardiac centers for interventional cardiology. This time frame has been demonstrated to decrease both morbidity and mortality.
The efficiency of this process can be substantially improved with 12-lead ECGs being obtained by Paramedics and the diversion of ambulances to cardiac centers. The American College of Emergency Physicians (ACEP) supports this process in their position paper entitled "Out-of-Hospital 12-Lead ECG."
While ACEP acknowledges that 12-lead ECG acquisition will prolong scene times, many Paramedics have become very adept at obtaining 12-lead ECGs in minimal time. Studies have shown that Paramedics can obtain 12-lead ECGs at the point of care in the field in approximately 5 minutes.
The advent of Paramedic 12-lead ECGs has greatly affected physicians’ opinions of Paramedics regarding the treatmet of acute coronary syndromes. Paramedics are viewed as a part of the continuum of care that starts in the field and ends in the interventional cardiologist suite. It is recognized that aggressive Paramedic care can substantially impact cardiac patient morbidity and mortality.
Origins of the Electrocardiogram
The electrocardiogram has a long history that may have started with Italian physicist Carlo Matteucci. Matteucci, interested in the works of the noted physicist Luigi Galvani, continued Galvani’s work on bioelectricity and started to investigate the role of electricity in the human body. Matteucci observed that with every heartbeat there was a passage of electrical current in the body. What Matteucci did not realize when he made that observation was that he was actually witnessing the birth of electrocardiography.
Following Matteucci’s early lead, noted British physiologist Augustus Waller, of St. Mary’s Medical School in London, created the first tracing of the heart’s electrical activity using his lab assistant Thomas Goswell as a patient in 1887. Subsequently, British physiologists William Bayliss and Edward Starling from the University College of London, following Waller’s direction, attached a terminal to the right hand of a patient and to the skin overlying the apex of the heart. They observed a triphasic pattern to the electricity’s flow.
Continuing the work of Bayliss and Starling, Willem Einthoven, who had also witnessed Waller demonstrate an ECG in 1889, used a silver string galvanometer to reproduce the triphasic waves that Bayliss and Starling had observed. Willem Einthoven named the deflections of these waves P, Q, R, S, and T, a convention that lasts to this day.
Continuing his work with the "electrokardiogram" (EKG) in 1905, Einthoven transmitted his first EKG over 1.5 kilometers to another lab using a telephone cable. This was the first recorded experience with telemetry.10 Einthoven also went on to standardize the electrocardiogram by referencing the body and using the designators Leads I, II, and III. These first leads formed an equilateral triangle which is now referred to as "Einthoven’s Triangle." From this platform, Einthoven was able to distinguish normal "EKG" from abnormal, noting premature ventricular contractions, heart blocks, atrial flutter, and other dysrhythmia. For this and other work, Einthoven was awarded the Nobel Prize in medicine in 1924 for "inventing the electrocardiogram." He is commonly referred to as the "father of electrocardiology."
Standard Limb Leads
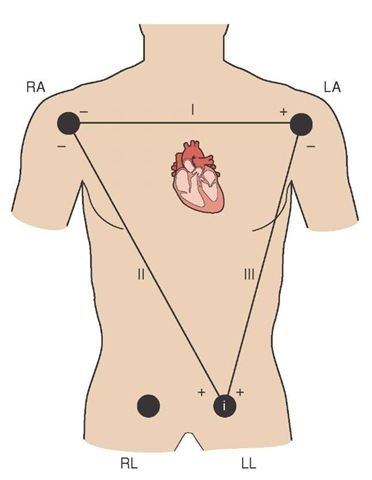
Einthoven’s limb leads used two electrodes, one negative and one positive, and measured the electrical potential between these electrodes as it flowed from negative to positive. Because the limb leads required two leads which measured the current difference between the leads’ electrodes, they were—and still are—referred to as bipolar leads. Einthoven’s original bipolar limb leads provided electrical information relating to the heart’s electrical activity along the frontal plane of the body (Figure 34-1).
Perhaps the most useful of these bipolar leads was Lead II in that its orientation, from right shoulder to the left foot, was more or less in alignment with the heart’s electrical conduction system. For this reason, Lead II provides the best view of error of conduction. It is often used to monitor patients for an irregular heart rhythm, a disturbance in conduction along the heart’s electrical pathway, called a dysrhythmia.
Unfortunately, because of its orientation along the frontal plane, Lead II only permits a view of the heart’s inferior wall. However, the bulk of the ventricular mass is in the anterior wall.11
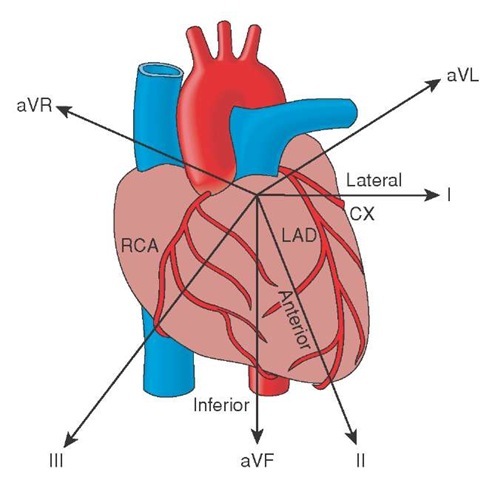
Even with the use of three limb leads, the ECG only viewed the frontal plane and the inferior and lateral wall of the heart (Figure 34-2). However, the majority of the ventricular mass lies along the transverse plane in the anterior wall. Thus, even with these additional leads, the electrical activity of the anterior wall was still not being captured by the ECG.
Precordial Leads
In an effort to obtain a more comprehensive view of the heart, researchers sought to create new leads. In 1931, researchers Wilson, MacLeod, and Barker devised a method for recording the electrical activity of the heart along any of its surfaces.
Figure 34-1 Standard limb leads.
Figure 34-2 Frontal plane’s relationship to the heart.
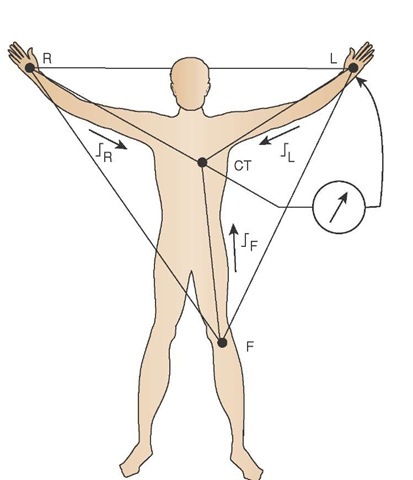
They continued to use the three limb leads. However, through the use of electrical resistors, they were able to move the negative electrode to the center of the body, proximal to the right atria and the SA node, to form a central point called Wilson’s central terminal (CT).
Because the negative electrode is a "virtual" electrode, not an actual electrode, in the center of the body, the positive electrode is now available to become an "exploring" lead which can be placed anywhere on the thorax to view any angle of the heart. The use of a single positive electrode, using Wilson’s central terminal, created the unipolar lead (Figure 34-3).
Figure 34-3 Wilson’s central terminal (i.e., the virtual negative electrode) and the creation of the unipolar lead.
Using the central terminal concept, Wilson placed electrodes in a semicircle around the precordium, that portion of skin that overlies the heart. Wilson’s unipolar precordial chest leads encircled the anterior ventricular wall from the septal wall on the right to the lateral wall on the left. This permitted a complete view of the anterior myocardium.
These leads were originally called the V leads (V for voltage). In 1938, the American Heart Association standardized the precordial leads and called them V1, V2, V3, V4, V5, and V6. Adding these precordial (chest) leads to the standard limb leads gave nine views of the heart along both the frontal plane and the transverse plane.
Augmented Leads— A More Complete Picture
While six precordial chest leads gave physicians a better view of the anterior wall, the three bipolar limb leads did not give physicians the same view of the inferior wall. Einthoven’s Triangle (the origin of the standard limb leads) is an equilateral triangle and has, by definition, angles of 60 degrees. Use of these angles left wide gaps with the potential for much of the heart’s electrical activity to be unrecorded by these standard limb leads.
Figure 34-4 Augmented lead placement and the relationship of augmented limb leads to the standard limb leads.
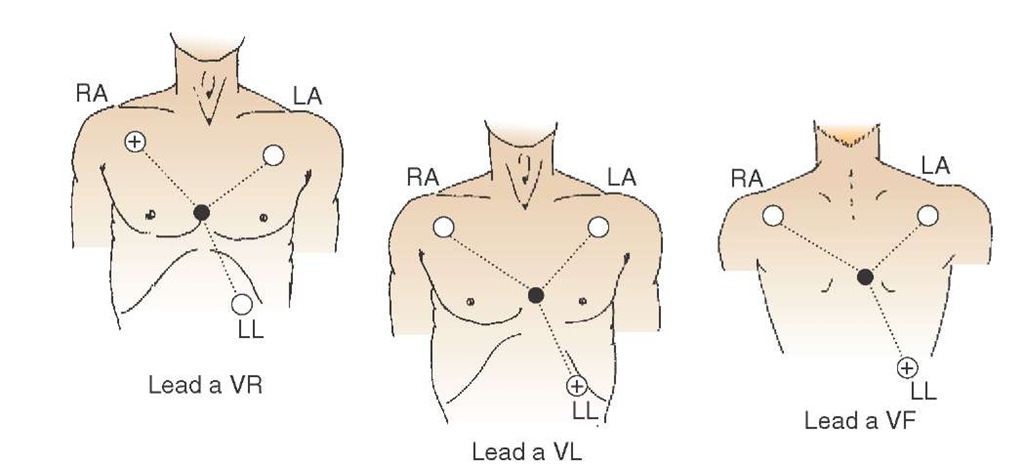
Using Wilson’s central terminal theory, Goldberger recorded the activity between each of the limb leads’ positive electrodes and the central terminal, adding three additional limb leads. Unfortunately, when the signals were recorded, they were too small to be properly examined and required a boosting of the signal in order to identify any characteristics. In 1942, Goldberger devised a method to boost, or augment, the signal (hence the title "augmented" leads) (Figure 34-4).
Combining the lead type and the positive electrode location gave rise to the names of Goldberger’s augmented leads (i.e., augmented voltage right or aVR, then augmented voltage left or aVL, and augmented voltage foot now called aVF).
Acquisition of the 12-Lead ECG
The importance of acquiring a high-quality 12-lead ECG, as explained earlier, cannot be overstated. Clinical decisions regarding the patient’s treatment and transportation are based, in part, on the 12-lead ECG. Therefore, it is imperative that the Paramedic know how to obtain a clear and concise 12-lead ECG.
Diagnostic 12-Lead ECG versus Rhythm Monitoring
Tracings of early ECGs were often plagued with artifact and distortions that made reading the ECG difficult. These early ECG machines were intended to monitor the rhythm only. To solve these problems, electrical engineers resorted to adjusting the frequency range, that area of the electric signal that is being recorded. They also used electronic filters such as common mode rejection.
In order to capture subtle changes in amplitude and duration which are necessary for the interpretation of a diagnostic 12-lead, the 12-lead ECG monitors require a wider frequency range than simple three-lead ECG rhythm monitors. In the simple ECG rhythm monitor, a narrower frequency range between 0.5 Hz and 30 to 50 Hz removes distortions which interfere with the rhythm’s correct analysis. However, it does so at the cost of accuracy when measuring segments of the ECG complex. In the simple ECG rhythm monitor, an ST segment may appear far above (elevated) or below (depressed) the baseline, indicating potential ischemia or acute injury/infarct. Paramedics are generally directed to take patients with an ST segment above baseline to an interventional cardiology center as opposed to the local hospital.12-15 A false positive in this regard is not only an inconvenience to the patient and to the patient’s family, but misuses critical cardiac services needed for more acutely ill patients.
To obtain a proper diagnostic 12-lead ECG that correctly shows all segments, the American Heart Association recommends that a frequency range of 0.05 Hz to 150 Hz be used. However, switching from monitoring mode to diagnostic mode raises the problems of artifact which were previously eradicated. Therefore, the Paramedic must take other measures to reduce artifact.
12-Lead ECG Artifact
The common sources of ECG artifact can be broadly classified as physiologic and nonphysiologic. Physiologic artifact includes muscle artifact and skin artifact. The first, muscle artifact, is the result of muscle movement or muscle tension. Muscle tension is the result of agonist and antagonist muscles competing to maintain a limb in one position. Any time a muscle contracts, it produces an electrical current which will be detected as an electromyographic signal (EMG) by the ECG. An EMG is seen as narrow rapid spikes on the ECG monitor.16
To prevent EMG on the ECG, the patient should be positioned comfortably, in an effortless position, with arms and legs supported by the stretcher. In some cases, it may be more prudent for the Paramedic to perform the ECG on the patient’s bed or couch rather than the stretcher because the patient’s arms hang off the stretcher. Folding of the arms across the chest only creates muscle tension and resultant EMG.
The other source of physiologic artifact is skin movement. Whenever the skin is stretched under an electrode, it will create an epidermal signal. For example, when a patient inhales and exhales, the movement of the skin will create baseline shifts (wandering baseline) as the electrodes move along with the chest wall. To prevent epidermal signal interference, it is important to properly place the electrodes on the patient’s chest away from the thoracic cage.
An example of a nonphysiologic source of ECG artifact is electromagnetic interference (EMI). Whenever alternating current (AC) electricity passes through a wire, it produces an electromagnetic field. Therefore, whenever a 12-lead ECG monitor is in the vicinity of a wire carrying AC electricity, the monitor will pick up the electromagnetic field as 60-cycle interference. An example of common sources of 60-cycle EMI are florescent lights, particularly those with a malfunctioning ballast, and poorly shielded ambulance convertors. Visually, 60-cycle EMI will present on the 12-lead ECG as a fuzzy baseline.
Static electricity can also produce artifact on the ECG. The patient may build up an electrical charge (static electricity). When the patient, as the charged body, is in proximity of an uncharged body, such as the ECG monitor, then electricity will pass between the two and be recorded on the ECG. This occurs frequently in dry climates.
One of the more common sources of artifact is electrode failure. Since the outer layer of skin is electrically "dead," an electrical signal cannot be transmitted across the skin without a conductive medium to act as a bridge between the inner body and the electrode. The typical ECG electrode uses silver chloride as the conductive medium. When a metal, such as silver chloride, is placed next to an electrolyte solution (i.e., the interstitial fluid), then an electromagnetic force is created and an electromagnetic "signal" is sent to the ECG monitor.
STREET SMART
Most 12-lead ECG cables are covered, or shielded, to prevent the monitor from picking up extraneous 60-cycle EMI. The presence of 60-cycle EMI on the monitor is a sign of either a defective cable or a broken lead.
If the skin is not properly prepared, then the skin can create an impedance to this signal of approximately 100,000 to 200,000 ohms. Simple site preparation can reduce the skin’s impedance to less than 10,000 ohms in 90% of patients and thereby markedly improve the quality of the ECG signal.
Patient Preparation
To prevent EMG interference, the Paramedic should first place the patient in a comfortable position. Preferably, the patient should be supine with arms at the sides and the entire body supported. If the patient is not relaxed, because of a painful condition such as arthritis, then the Paramedic might consider using analgesia or sedatives.
The Paramedic should then prepare the skin before applying the electrodes. If the patient’s chest hair interferes with the Paramedic’s attempts to closely adhere the electrodes to the skin, then the chest hair needs to be removed. While it might be expeditious to use a straight razor to remove the hair, shaving the chest can create microlacerations that can bleed if the patient is given fibrinolytics later. Also, the hair follicles can become infected (folliculitis). The patient’s chest hair should be carefully trimmed using either a commercially available clipper or a pair of blunt tipped bandage scissors.
STREET SMART
If the patient is grossly diaphoretic, some Paramedics have used antiperspirants to dry the area. These antiperspirants often contain aluminum oxide as an active agent. Aluminum oxide interferes with the electrical signal and will reduce the quality of the 12-lead ECG.
While wiping the contact surface with a gauze pad will reduce the pickup of 60 Hz EMI and motion artifact, it will only reduce the skin’s resistance by 1,000 to 5,000 ohms. It is important to not only remove the dead cells of the stratum corneum but to also scratch the lower stratum granulosum.17
Scratching the stratum granulosum improves the ECG signal by allowing the electrode gel to permeate the skin and contact the electrolyte solution (i.e., interstitial fluid). Typically, either fine weight sandpaper (220 to 3,400 grit) or a commercially available gritty ECG preparation gel is used. Five to ten strokes of either a gel-soaked gauze pad or sandpaper is sufficient. The skin should be slightly reddened but not abraded.
The area immediately surrounding the electrode contact should be cleansed with an alcohol-soaked preparation pad. The alcohol helps to remove surface fats which can undermine the electrode’s adhesive and prevent close skin contact with the electrode’s gel.
It is important that the gel on the electrode be moist. Most electrodes come prepackaged and have an expiration date. The Paramedic should first confirm that the electrodes are not expired before proceeding. Next, the Paramedic should remove the electrode from the package and remove the plastic protective cover over the gel. With one finger, the Paramedic should gently compress the gel. Moist electrode gel should have a little spring when gently compressed. Dried electrode gel will be stiff and unyielding. Dried electrodes should be discarded immediately as they are of no practical use.
Electrode gels depend on the body’s warmth to liquefy the gel so that it can penetrate the skin. If the patient’s skin is cold, then the gel will not melt and the ECG signal will be poor. Waiting until the patient is warmed, or applying hot packs over the electrodes, can improve the quality of the ECG.
After confirming that the gel on the electrode is still moist, the lead wire should be attached to the electrode. The gel under the electrode is formed into a pod so that the gel stays concentrated in an area when the electrode is then placed on the skin. If the electrode is placed on the skin first and then the lead wire is attached, the pressure from attaching the lead wire can crush the pod, disperse the gel, and diminish the signal quality.
If moisture or blood is expected to cover the electrode, the application of bio-occlusive dressing over the electrode can prevent the moisture from undermining the electrode and interrupting the ECG signal.