By : James W Zubrick
Email: j.zubrick@hvcc.edu
Maximum-Boiling Azeotropes
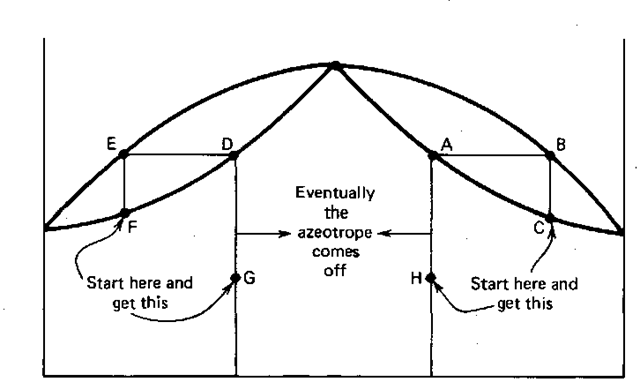
The chloroform-actone azeotrope (52%chloroform-48%acetone) is an example of the much rarer maximum boiling azeotrope. It’s boiling point is higher than that of the components (Fig. 144). At compositions off the azeotrope, you do distillations A-B-C or D-E-F (and so on) until the boiling liquid composition reaches the azeotropic composition. Then that’s all that comes over. So, initially, one of the components comes off first.
Azeotropes on Purpose
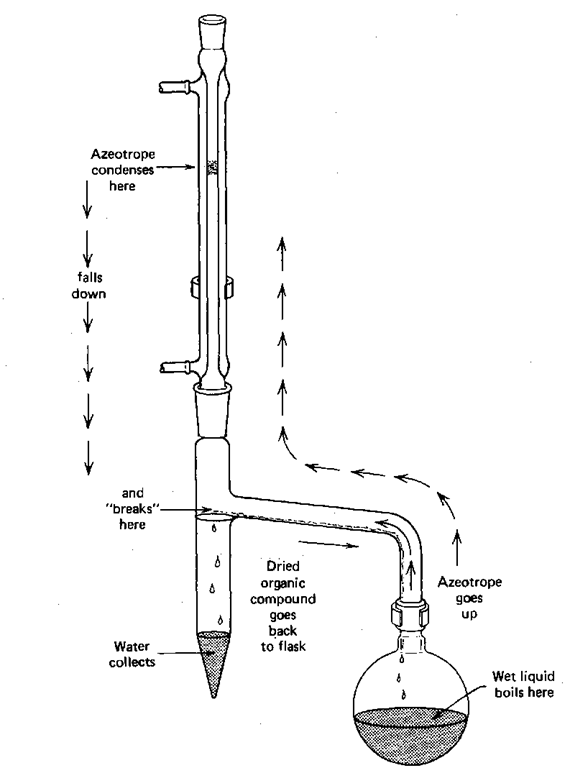
You might think the formation of azeotropes to be an unalloyed nuisance, but they can be useful. Toluene and water form a minimum-boiling azeotrope (20.2%water; 85°C). If you needed very dry toluene for some reason, all you need to do is distill some of it. The water-toluene azeotrope comes off first, and, well, there goes all the water. It gets removed by azeotropic distillation. The technique can also be used in certain reactions, including the preparation of amides from very high boiling acids and amines (they can even be solids.). You dissolve the reagents in toluene, set up a reflux condenser fitted with a Dean—Stark trap, (Fig. 145) and let the mixture reflux. As the amide forms, water is released and the water is constantly removed by azeotropic distillation with the toluene. The azeotrope cools, condenses, and collects in the Dean – Stark trap. At room temperature, the azeotrope is said to “break,” and the water forms a layer at the bottom of the trap. Measure the amount of water and you have an idea of the extent of the reaction.
Fig. 144 Maximum-boiling chloroform-acetone azeotrope.
Absolute (100%) ethanol is often made by adding benzene to the ethanol-water binary azeotrope (two components), to make a ternary azeotrope (three components). This ternary alcohol-water-benzene (18.5:7.4:74.1) azeotrope comes over until all the water is gone, followed by a benzene-ethanol mixture. Finally, absolute ethanol gets its chance to appear, marred only slightly by traces of benzene.
Other Deviations
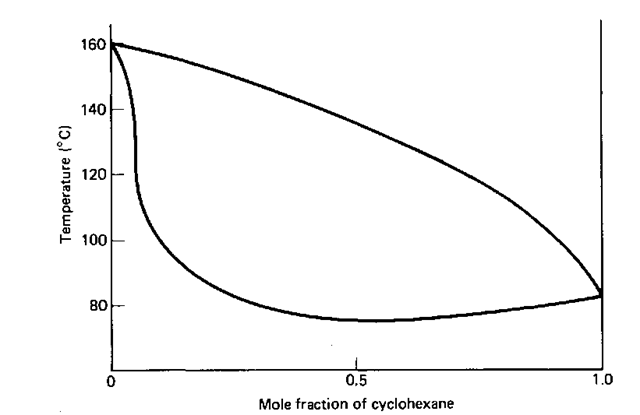
The furfural -cyclohexane phase diagram (Fig. 146) shows that you can have mixtures that exhibit nonideal behavior, without having to form an azeotrope. In sum, without the phase diagram in front of you, you shouldn’t take the distillation behavior of any liquid mixture for granted.
CLASS 4: STEAM DISTILLATION
Steam distillation is for the separation of mixtures of tars and oils, and they must not dissolve much in water. If you think about it a bit, this could be considered a fractional distillation of a binary mixture with an extreme deviation from Raoult’s Law. The water and the organic oils want nothing to do with each other. So much so, that you can consider them unmixed, in separate compartments of the distilling flask. As such, they act completely independently of each other. The mole fraction of each component in its own compartment is 1. So Raoult’s Law becomes
This is just Dalton’s Law of partial pressures.![]() for a steam distillation. So the vapor pressure of the organic oil is now less than that of the atmosphere and the water, and codistills at a much lower temperature.
for a steam distillation. So the vapor pressure of the organic oil is now less than that of the atmosphere and the water, and codistills at a much lower temperature.
Fig. 145 Removing water by azeotropic distillation.
Fig. 146 Other deviant behavior (but no azeotropes) in the furfural-cyclohexane system.
As an example, suppose you were to try to directly distill quinoline. Quino-line has a boiling point of 237 °C at 1 atm. Heating organic molecules to these temperatures may often be a way to decompose them. Fortunately, quinoline is insoluble in water and it does have some vapor pressure at about the boiling point of water (10 torr at 99.6°C). If it had a much lower vapor pressure at the boiling point of water, (say 0.1 torr), there wouldn’t be enough of it vaporizing to make even steam distillation worthwhile.
Well, at 99.6 °C, quinoline contributes 10 torr to the total vapor pressure, and water must make up the difference (750 torr) in order to satisfy Dalton’s Law of partial pressures to make PTota1760 torr at boiling. Using the relationships for the composition of the vapor over a liquid, we can calculate the quinoline/water ratio coming over.
The number of moles (n) of anything is just the weight in grams (g) divided by the molecular weight of that substance (MW), and so
If we consider each to be an ideal gas, then
and isolating the mass of the material by dividing by RT gives
Now for two vapors, A and B, I’ll construct a ratio where
Multiplication by MW gives
With A and B in the same flask, R, T, and V must be the same for each, and we can cancel these terms giving
If we plug in values for the molecular weights and vapor pressures of quinoline and water, we get
So, as an approximation, for every 10 g of distillate we collect, 1 g will be our steam distilled quinoline.
![tmp170-123_thumb[2][2] tmp170-123_thumb[2][2]](http://what-when-how.com/wp-content/uploads/2011/06/tmp170123_thumb22_thumb.png)
![tmp170-125_thumb[2][2] tmp170-125_thumb[2][2]](http://what-when-how.com/wp-content/uploads/2011/06/tmp170125_thumb22_thumb.png)
![tmp170-126_thumb[2][2] tmp170-126_thumb[2][2]](http://what-when-how.com/wp-content/uploads/2011/06/tmp170126_thumb22_thumb.png)
